δ 18 O
δ 18 O or Delta-O-18 is a measure of the ratio of the stable oxygen - isotopes 18 O and 16 O. The determination of the ratio is a sub-discipline of isotope geochemistry and is carried out, among other things, in the context of petrological , stratigraphic or paleoclimatological investigations. δ 18 O values in certain minerals of igneous rocks reflect the temperature of the magma from which the minerals are crystallized, or they give indications of isotope fractionation as a result of reactions of a crystallized rock with aqueous solutions. δ 18 O values in the carbonate minerals or the opal of a wide variety of macro and microfossils , for example diatoms in freshwater lake sediments, are used as a temperature proxy.
The δ 18 O value is defined as:
The unit is per thousand (‰, parts per thousand). The standard has a known isotopic composition such as B. the Vienna Standard Mean Ocean Water (VSMOW).
Mechanism and application
δ 18 O also reflects the local evaporation rate and the fresh water influx. Since 16 O preferably evaporates from seawater , rainwater is rich in 16 O. As a result, the surface water of the oceans in the subtropics contains larger amounts of 18 O, because the rate of evaporation is increased there. Lower amounts of 18 O are found in mid-latitude ocean water, where it rains more.
Something similar can be observed with condensation: water molecules, which contain the heavier 18 O atoms, tend to be the first to condense and rain out. The gradient of the water moisture shows a decrease in the 18 O content from the tropics to the poles . Snow falling in Canada contains less H 2 18 O than rain falling in Florida ; accordingly, snow falling in the center of an ice surface has a lighter δ 18 O signature than on the edges of the ice surface, since the heavier 18 O rains down first. A climate change that changes the global pattern of evaporation and precipitation changes the fundamental δ 18 O ratio for this reason .
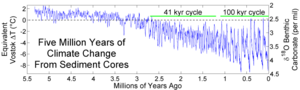
These connections were discovered and published by Harold C. Urey in 1947. Building on this, Cesare Emiliani analyzed large amounts of drill cores in the 1950s and subdivided the geological epochs into so-called oxygen isotope levels based on the δ 18 O signatures found .
Appropriate proxies
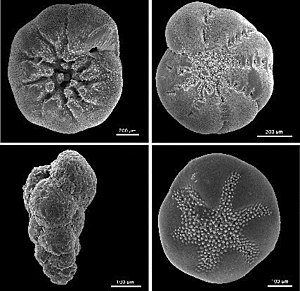
The shells of foraminifera were examined most frequently . Made from calcium carbonate (CaCO 3 ), they can be found in many geological structures, and contain oxygen. The ratio 18 O to 16 O is used to indirectly determine the temperature of the surrounding water at the time of crystallization. The ratio varies slightly depending on the temperature of the surrounding water; but other factors also influence the ratio such as B. the salinity and the amount of water trapped in ice sheets. However, reconstructions are also possible using corals , lake sediments and stalagmites . In addition, the ice from ice cores and the sugar of fossil plants can be examined.
While the suitability of δ 18 O from ice core samples for the reconstruction of temperatures of past times has been proven many times, it can be with a temperature reconstruction in which biogenic phosphate material such. B. bone and tooth material is used, lead to falsifications by diagenesis .
Analysis of sugar
In the sugars ( arabinose , xylose and fucose ) of plants deposited in lake sediments, a clear δ 18 O signature can also be recognized with the help of a new method , which probably reflects the local temperature or precipitation situation. In the Himalayan region, for example, this signature can be used as a proxy for the intensity and variability of the summer monsoon .
Review of food
Another application is checking the authenticity of food. Since water absorbed by plants is stored in water, every vegetable food bears the signature of its place of growth in the water it contains. If, for example, wine is diluted with tap water or juice, which has previously been converted into a concentrate for inexpensive transport, is re-diluted with tap water, this can be detected by determining δ 18 O. If sufficiently precise data is available about a cultivation area, it is possible with the procedure to also check information on the cultivation location. For example, it can be checked whether a Bordeaux wine actually comes from grapes that grew in Bordeaux .
Calculations
If the influence of changing salinity and ice volume are disregarded and the signal is consequently attributed exclusively to temperature changes, an increase in δ 18 O by 0.22 ‰ corresponds to a cooling of 1 K.
The temperature can also be calculated using this equation:
During the Pleistocene , a δ 18 O signature of 0.11 ‰ correlated with a sea level change of 10 m, which resulted from the change in ice volume.
See also
literature
- Clark, ID and Fritz, P: Environmental Isotopes in Hydrogeology . CRC Press , 1997, ISBN 1-56670-249-6 .
- Schmidt, GA: Forward Modeling of Carbonate Proxy Data from Planktonic Foraminifera Using Oxygen Isotope Tracers in a Global Ocean Model . In: Paleoceanography . 14, No. 4, 1999, pp. 482-497. bibcode : 1999PalOc..14..482S . doi : 10.1029 / 1999PA900025 .
Individual evidence
- ↑ Lorraine E. Lisiecki, Maureen E. Raymo: A Pliocene-Pleistocene stack of 57 globally distributed benthic δ 18 O records . In: Paleoceanography . 20, No. 1, March 2005, pp. N / a – n / a. ISSN 0883-8305 . doi : 10.1029 / 2004PA001071 .
- ↑ Melanie J Leng, Jom D Marshall: Palaeoclimate interpretation of stable isotope data from lake sediment archives . In: Quaternary Science Reviews . 23, No. 7-8, April 2004, pp. 811-831. doi : 10.1016 / j.quascirev.2003.06.012 .
- ↑ USGS - Isotope Tracers - Resources - Isotope Geochemistry . Retrieved January 18, 2009.
- ↑ Harold C. Urey: The thermodynamic properties of isotopic substances . In: Journal of the Chemical Society (Resumed) . 1947, p. 562. ISSN 0368-1769 . doi : 10.1039 / JR9470000562 .
- ↑ J. Jouzel , Richard B. Alley , KM Cuffey, Willi Dansgaard , P. Grootes, G. Hoffmann, SJ Johnsen, RD Koster, D. Peel, CA Shuman, M. Stievenard, M. Stuiver, J. White: Validity of the temperature reconstruction from water isotopes in ice cores . In: Journal of Geophysical Research: Oceans . 102, No. 26471 - 26487, September 2012. doi : 10.1029 / 97JC01283 .
- ↑ ZD Sharp, V. Atudorei and H. Furrer: The effect of diagenesis on oxygen isotope ratios of biogenic phosphates . In: American Journal of Science . 300, No. 3, March 2000, pp. 222-237. doi : 10.2475 / ajs.300.3.222 .
- ↑ Michael Zech, Mario Tuthorn, Roland Zech, and Frank Schlütz, Wolfgang Zech , Bruno Glaser : A 16-ka δ18O record of lacustrine sugar biomarkers from the High Himalaya reflects Indian Summer Monsoon variability . In: Journal of Paleolimnology . 50, No. 2, August 2013, pp. 1–11. doi : 10.1007 / s10933-013-9744-4 .
- ↑ Editor LGL: Stabilisotopeanalytik . In: Food labeling, protection against deception . Bavarian State Office for Health and Food Safety. March 22, 2012. Retrieved July 6, 2013.
- ^ Visser, K., Robert Thunell, Lowell Stott: Magnitude and timing of temperature change in the Indo-Pacific warm pool during deglaciation . In: Nature . 421, No. 6919, 2003, p. 152. doi : 10.1038 / nature01297 .