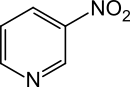
3-nitropyridine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 3-nitropyridine | |||||||||||||||
| Molecular formula | C 5 H 4 N 2 O 2 | |||||||||||||||
| Brief description |
yellow solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 124.10 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.33 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
38-42 ° C |
|||||||||||||||
| boiling point |
216 ° C |
|||||||||||||||
| solubility |
slightly soluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
3-Nitropyridine is a heterocyclic chemical compound . The structure is a pyridine ring which has a nitro group in the 3-position .
presentation
3-Nitropyridine cannot be obtained under the usual aromatic electrophilic nitration conditions. However, the conversion of pyridine with nitrous oxide to 3-nitropyridine has been reported several times . It can also be made from 2,6-dibromopyridine with nitronium tetrafluoroborate and subsequent dehalogenation.
Individual evidence
- ↑ a b c d e f g Data sheet 3-Nitropyridine, 97% from AlfaAesar, accessed on December 25, 2019 ( PDF )(JavaScript required) .
- ↑ Jan M. Bakke, Ingrid Hegbom: "Dinitrogen Pentoxide – Sulfur Dioxide, a New Nitration System", in: Acta Chemica Scandinavica , 1994 , 48 , pp. 181-182; doi : 10.3891 / acta.chem.scand.48-0181
- ↑ Jan M. Bakke, Eli Ranes: "A New Efficient Synthesis of 3-Nitropyridine and Substituted Derivatives", in: Synthesis , 1997 , 3 , pp. 281-283; doi : 10.1055 / s-1997-4463 .
- ↑ Takashi Murashima, Keiji Nishi, Ken-ichi Nakamoto, Atsushi Kato, Ryuji Tamai, Hidemitsu Uno, Noboru Ono: "Preparation of Novel Heteroisoindoles from Nitropyridines and Nitropyridones", in: Heterocycles , 2002 , 58 , pp. 301-310; doi : 10.3987 / COM-02-S (M) 22 .
- ↑ Joseph L. Duffy, Kenneth K. Laali: "Aprotic Nitration (NO 2 + BF 4 - ) of 2-Halo- and 2,6-Dihalopyridines and Transfer-Nitration Chemistry of Their N -Nitropyridinium Cations", in: J. Org. Chem. , 1991 , 56 , pp. 3006-3009; doi : 10.1021 / jo00009a015 .

