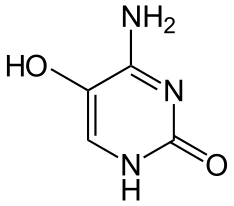
5-hydroxycytosine
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||
| General | |||||||||||||
| Surname | 5-hydroxycytosine | ||||||||||||
| other names |
4-Amino-5-hydroxypyrimidin-2 (1 H ) -one |
||||||||||||
| Molecular formula | C 4 H 5 N 3 O 2 | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 127.10 g mol −1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
5-Hydroxycytosine is a heterocyclic organic compound with a pyrimidine backbone. It is a derivative of the nucleic base cytosine with an additional hydroxyl group in position 5. It has been shown experimentally that it is removed from damaged DNA by endonuclease III in E. coli . It arises from the dehydration of the cytosine glycol produced by the oxidation of cytosine. This DNA mutation occurs more frequently in people with Alzheimer's disease than in people who are not.
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Erich Schiller: Free Radicals and Inhalation Pathology Respiratory System, Mononuclear Phagocyte System, Hypoxia and Reoxygenation, Pneumoconioses, and Other Granulomatoses Cancer . Springer Science & Business Media, 2004, ISBN 978-3-540-00201-7 , pp. 710 ( limited preview in Google Book search).
- ^ Karl E. Zahn, April Averill et al. a .: The Miscoding Potential of 5-Hydroxycytosine Arises Due to Template Instability in the Replicative Polymerase Active Site. In: Biochemistry . 50, 2011, pp. 10350-10358, doi: 10.1021 / bi201219s .
- ^ J. Richard Wagner, Benjamin C. Blount, Michael Weinfeld: Excision of Oxidative Cytosine Modifications from γ-Irradiated DNA by Escherichia coli Endonuclease III and Human Whole-Cell Extracts , Analytical Biochemistry , 233 (1), 1996, p. 76– 86, doi: 10.1006 / abio.1996.0010 .
- ↑ Varatharasa Thiviyanathan, Anoma Somasunderam, David E. Volk, Tapas K. Hazra, Sankar Mitra: Base-pairing properties of the oxidized cytosine derivative, 5-hydroxy uracil . In: Biochemical and Biophysical Research Communications . tape 366 , no. 3 , February 2008, p. 752-757 , doi : 10.1016 / j.bbrc.2007.12.010 , PMID 18078807 , PMC 2262052 (free full text).
- ↑ S. Prasad Gabbita, Mark A. Lovell, William R. Markesbery: Increased Nuclear DNA oxidation in the brain in Alzheimer's Disease . In: Journal of Neurochemistry . tape 71 , no. 5 , 1998, pp. 2034-2040 , doi : 10.1046 / j.1471-4159.1998.71052034.x .