Alfuzosin
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
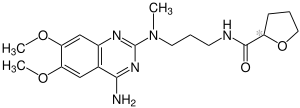
|
||||||||||||||||||||||
| Simplified structural formula without stereochemistry | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Alfuzosin | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula |
|
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| Mechanism of action |
α 1 adrenergic receptor antagonist |
|||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | ||||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
225 ° C ( hydrochloride ) |
|||||||||||||||||||||
| pK s value |
8.13 |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data |
|
|||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Alfuzosin is a drug from the group of α 1 -adrenoreceptor antagonists ( alpha blockers ), which is used in the treatment of benign prostatic hyperplasia (BPH). The chiral compound is derived from quinazoline . It was patented by Synthélabo (now Sanofi ) as an antihypertensive agent in 1979 and 1982 .
Clinical information
application areas
The drug is used to treat the symptoms of benign prostatic hyperplasia. It has no influence on the size of the prostate .
Contraindications (contraindications)
In the presence of severe hepatic insufficiency or orthostatic hypotension (low blood pressure), administration is contraindicated, as is treatment with other alpha blockers.
Adverse effects (side effects)
Possible side effects include fatigue, dizziness, headache, flu symptoms, and hypotonic dysregulation.
Pharmacological properties
As an antagonist, alfuzosin binds selectively to postsynaptic α 1 -adrenoreceptors and thus relaxes the smooth muscles of the prostate and urethra. This increases the flow of urine and facilitates urination . The bioavailability is 64% and the plasma half-life is 4 to 6 hours. The maximum plasma concentration is reached after 90 minutes.
Stereochemistry
Alfuzosin contains a stereocenter and is therefore chiral . There are two enantiomeric forms, ( R ) -form and ( S ) -form. However, only the racemate [( RS ) -Alfuzosin], a 1: 1 mixture of the ( R ) -enantiomer and the ( S ) -enantiomer, has practical significance :
| Alfuzosin enantiomers | |
|---|---|
 ( R ) -Alfuzosin |
 ( S ) -Alfuzosin |
Trade names
Alfunar (D), Fuzocim (CH), Urion (D), UroXatral (D), Xatral (CH)
Individual evidence
- ↑ a b c Entry on alfuzosin. In: Römpp Online . Georg Thieme Verlag, accessed on September 9, 2014.
- ↑ a b c d Datasheet Alfuzosin hydrochloride from Sigma-Aldrich , accessed on September 9, 2014 ( PDF ).
- ↑ a b c Michael C. Truß, Christian G. Stief, Stefan Machtens, Till Wagner, Udo Jonas: Pharmacotherapy in urology. 2nd, completely revised edition. Springer, Heidelberg 2005, ISBN 3-540-23449-7 , p. 302 ff.
- ↑ Rote Liste Service GmbH (Ed.): Rote Liste 2017 - drug directory for Germany (including EU approvals and certain medical devices) . Rote Liste Service GmbH, Frankfurt / Main, 2017, edition 57, p. 159, ISBN 978-3-946057-10-9 .
- ↑ ROTE LISTE 2017, Verlag Rote Liste Service GmbH, Frankfurt am Main, ISBN 978-3-946057-10-9 , p. 159.