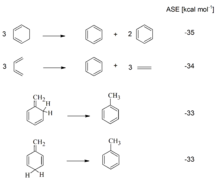Aromaticity
Aromaticity is a concept from the field of chemical bonding that was introduced to explain the striking properties of the class of aromatics . It describes the phenomenon that cyclic delocalization of 4n + 2 π electrons ( Hückel rule ) leads to an energetic stabilization in a planar molecule, which occurs with the occurrence of diamagnetic ring current effects, anisotropy of magnetic susceptibility , magnetic susceptibility exaltation and a tendency to Bond length compensation is associated. The phenomenon of aromaticity is not a summary observable , but the individual criteria are measurable. However, for the individual criteria no fixed or clear limit values for the distinction between aromatic, non- aromatic and anti-aromatic are set. The discussion about the extent of the aromaticity of individual compounds usually takes place with reference to a reference. The following applies benzene controversy as a prime example of an aromatic system.
While the discussion of aromaticity was originally limited to cyclically conjugated π systems, the term aromaticity is now much broader and expanded to include homoaromaticity , σ-aromaticity , σ-homoaromaticity , spatial aromaticity , superaromaticity, etc. In particular, the development of quantum chemical methods has significantly supported the expansion of theoretical understanding.
If instead of 4n + 2 electrons 4n electrons are cyclically delocalized in a molecule, the phenomenon of antiaromaticity is observed . In this case, the system is destabilized. Antiaromatic systems will try to avoid the unfavorable situation and to reduce the cyclic delocalization through geometric changes (distortion, Jahn-Teller effect ). A prime example of this is cyclobutadiene .
The following table summarizes the characteristics of aromatic and anti-aromatic systems compared to linear conjugated references:
| property | Aromatic | Reference (olefin) | Anti-aromatic |
|---|---|---|---|
| Delocalization / conjugation | cyclic | linear | cyclic |
| Number of π electrons | 4n + 2 | 2n | 4n |
| Energetic effect of conjugation | stabilization | = Reference | destabilization |
| Extent of delocalization | elevated | = Reference | humiliated |
| Bond lengths | Tendency to Binding length compensation |
alternating | alternating |
| Diamagnetic anisotropy | elevated | - | small |
| magnetic susceptibility exaltation | high | - | low |
| Ring current | diamagnetic | - | paramagnetic |
| NICS values | clearly negative | - | clearly positive |
| Chemical reactivity | electrophilic substitution | addition | addition |
| HOMO-LUMO difference | elevated | = Reference | humiliated |
| Typical representatives | benzene | Butadiene | Cyclobutadiene |
Development of the concept of aromaticity
Aromaticity was initially described in terms of olfactory and chemical properties: compounds that smelled particularly “aromatic” showed astonishingly low reactivity for unsaturated compounds. So z. B. bromine does not spontaneously add to the double bonds of benzene. Benzene and its derivatives also react preferentially according to the pattern of electrophilic aromatic substitution (addition-elimination mechanism, which leads to the maintenance of cyclic delocalization) and not with simple addition to one of the (formal) double bonds. While early work relied on synthetic methods - e.g. B. Attempts to prove the cyclic structure of benzene by means of the number of isomers and to clarify the question of whether there is an alternation of double and single bonds - more and more physical aspects became in the course of the development of the (electron) theory of chemical bonds included the investigations. An exhaustive compilation of important milestones in the development of the concept of aromaticity and the criteria for defining aromaticity can be found in Schleyer et al. - Some key aspects can be found in the following table:
| year | aspect |
|---|---|
| 1865 | Kekulé: benzene structure |
| 1866 | Erlenmeyer: The predominance of electrophilic substitution instead of addition |
| 1910 | Pascal: Aromatics have increased diamagnetic susceptibility |
| 1925 | Armit & Robinson: Electron Sextet and Heteroaromaticity |
| 1931 | Hückel: Stabilization of the 4n + 2-, destabilization of the 4n electron systems (Hückel rule) |
| 1936/1937 | London, Pauling: Theory of the ring current effects |
| 1956 | Pople: Influence of ring current effects on NMR shifts |
| 1969/1970 | Dauben / Benson & Flygare: magnetic susceptibility (increase and anisotropy) as aromaticity criteria |
| 1979 | Aihara: three-dimensional aromaticity |
| 1980 | Kutzelnigg: Quantum chemical calculation (IGLO) of NMR and magnetic molecular properties |
| 1984 | Dewar: σ-aromaticity of cyclopropane |
| 1996 | Schleyer: Introduction of NICS as a simple quantum chemical method to study aromaticity |
For an overview of the early history of the study of aromatics, cf. z. B. under benzene .
Types of aromaticity
- π aromaticity
In the archetypal case of aromaticity, cyclic delocalization occurs through conjugation of π orbitals along a closed perimeter, which is defined by σ bonds.
- Hetero-aromaticity
Starting from benzoid aromatics, individual carbon atoms are replaced by heteroatoms. Pyridine , thiophene , furan , pyrrole are typical examples.
- σ-aromaticity
With small rings (especially with cyclopropanes ), cyclic delocalization is observed in the σ-plane. As with π-aromaticity, energetic stabilization and the magnetic properties typical of diamagnetic ring currents are observed. In purely inorganic systems, the cyclic ion H 3 + is a prime example of σ-aromaticity. Σ-aromaticity is also discussed for trigonal metal clusters of Zn and Au.
- Homoaromaticity
If, in the case of π-aromatics, the closed perimeter of σ-bonds is interrupted at one point, e.g. B. by introducing a methylene group, so the π-conjugation through space ( through space ) can be maintained at this position with sufficient geometric conditions . The homotropylium cation is a prime example of this phenomenon. If the perimeter is interrupted in several places, one speaks of Bishomo, Trishomo aromaticity, etc.
- σ-homoaromaticity
As with the π aromatics, the σ conjugation can take place through space with small rings . Examples of σ-bishomoaromaticity in 2- and 6-electron systems are described.
- Spherical Aromaticity (Spatial Aromaticity, 3D Aromaticity)
Aromaticity in three dimensions is described for cluster compounds such as boranes and fullerenes .
Aromatic stabilization energy
An unusual feature of benzene that was known early on was the fact that, in contrast to other unsaturated compounds, benzene is comparatively difficult to hydrogenate. In thermochemical measurements, it was also found that the heat of hydrogenation released in the case of benzene is significantly reduced compared to a hypothetical non-aromatic cyclohexatriene. An aromatic stabilization energy of benzene ( ASE ) of −150 kJ / mol (−36 kcal / mol) was derived from this difference . (Unfortunately, the sign convention of the ASE is not always strictly adhered to in the literature. In this article a negative ASE (stabilization!) And a positive ASE (destabilization!) For antiaromatic compounds is used for aromatic compounds.)
In addition to thermochemical measurements, a large number of quantum chemical methods are now available to estimate aromatic stabilization energies. Usually this is done using homodesmotic reactions. By using homodesmotic reaction equations, inaccuracies resulting from the quality of the calculation methods used should be averaged out so that the variable of interest is obtained with high accuracy. Regardless of whether the estimation of the aromatic stabilization energy is based on thermochemical measurements or calculations, it must always be taken into account that the aromatic stabilization energy is not an observable. The ASE therefore depends on the selected reference connections and is also not easy to separate from other effects - such as ring stresses . Stabilization effects due to resonance must also be calculated that are not based on cyclic delocalization and thus contribute to the total resonance energy but not to the ASE. These problems are already evident in the calculation of the ASE of benzene: even with careful selection of the reference structures for homodesmotic reactions, the calculated values fluctuate slightly by 10 kcal mol −1 , as a compilation by Cyrański shows. To get around the problem, Schleyer et al. propose to use isomerization reactions instead of homodesmotic reaction schemes to determine the ASE. The calculated isomerization stabilization energies ( ISE ) are in good agreement with comparative values from carefully selected homodesmotic reaction schemes.
A number of Schleyer et al. ASEs for 5-membered ring compounds calculated on the basis of a "homodesmotic" reaction confirm both the Hückel rule and the phenomenon of heteroaromaticity, which was known early on:
| ASE [kcal mol −1 ] | |||||||
|---|---|---|---|---|---|---|---|
| X | CH | CH 2 | SiH | bra | NH | O | S. |
| Name of C 4 H 4 X | Cyclopentadiene | Borol | Pyrrole | Furan | Thiophene | ||
| cation | 57 | 24 | |||||
| Neutral connection | −4 | 19th | −26 | −20 | −22 | ||
| Anion | −29 | −14 | |||||
The criterion of the aromatic stabilization energy follows the "practical experience" of chemists that aromatic compounds are characterized by particular stability (negative value for the ASE). The values given above for antiaromatic systems (table, 5-ring systems with 4n π electrons) are in agreement with this (destabilization by antiaromaticity). Cyclobutadiene, a prime example of antiaromaticity, finds out after the assessment
- Cyclobutadiene + cyclobutane → 2 cyclobutene
a destabilization of +36 kcal mol −1 .
Impressive examples of the fact that cyclic conjugation of 4n + 2 electrons leads to stabilization and cyclic conjugation of 4n electrons to destabilization are provided by isodesmic reactions of cyclopropene or cyclopentadiene with cyclopropyl or cyclopentyl anions and cations (cf. Illustration). The formation of aromatic ions is indicated by high exothermicity, the formation of antiaromatic ions by clear endothermia.
NMR effects, NICS
In NMR experiments, the effect of cyclic electron delocalization in aromatic compounds can be demonstrated by the accompanying magnetic effects (ring current effect): the magnetic field induced by the ring current leads to a proton resonance of 7.3 ppm (deshielding) in benzene, whereas comparable vinylic protons appear in cyclohexene at 5.6 ppm. Protons that are located above or in the inner area of an aromatic π system, on the other hand, are shielded. Textbook examples for this effect are e.g. B. the methylene protons in methano [10] annulene ( Vogel's aromatic ) or the inner protons in [18] annulene .
In addition to the proton resonances, the chemical shifts of Li + were also frequently used to study the aromatic properties of anionic π systems, since lithium usually binds electrostatically in a central position above the π system and is therefore a less susceptible sample for the magnetic properties of the to the species under investigation. Typical examples of this approach can be found in the Li salts of the (di) anions of cyclopentadiene, tetrakis-trimethylsilyl-cyclobutadiene and the antiaromatic dianion of tetrakis-trimethylsilyl-benzene (see figure).
With the introduction of quantum chemical methods, it became possible to calculate chemical shifts (shielding) at any point in space and thus to evaluate ring current effects independently of individual atomic nuclei. The method was named by its protagonists Nucleus Independent Chemical Shifts (NICS). To study aromatic compounds, NICS values are usually calculated in the geometric center of the heavy atoms (ie the non-hydrogen atoms, not mass averaged). As usual in the case of protons, negative NICS values in the interior of cyclically delocalized systems indicate a diamagnetic ring current and thus aromaticity. Positive NICS values, on the other hand, indicate antiaromaticity.
Although the cyclic electron delocalization dominates the magnetic properties of (anti) aromatic molecules, localized bonds and ion pairs also show at least short-range magnetic effects, which can influence the calculated NICS values. In order to eliminate this interference factor, NICS values above the π-plane can be calculated for planar molecules. More complex methods also allow NICS contributions from σ and π bonds or from individual molecular orbitals to be calculated and thus separated.
Magnetic susceptibility
The magnetic susceptibility χ is a quantity known from physics and describes how strongly a substance can be magnetized when it is introduced into a magnetic field. The magnetic susceptibility can be traced back to contributions along the three spatial directions (x, y, z) and is mostly used in chemistry as a molar quantity. For disordered liquids, the observable susceptibility results as the mean value from the three susceptibilities of the molecules along the spatial directions:
- χ M = 1/3 (χ x + χ z + χ z )
If benzoid aromatics are oriented such that the molecule lies in the xy plane, the magnetic susceptibility in the z direction is significantly greater than within the molecular plane. This phenomenon is called increased diamagnetic anisotropy and is described by the following equation:
- Δχ = χ z - 1/2 (χ x + χ y ) >> 0
Since the experimental determination of the anisotropic properties is difficult and can only be carried out on single crystals, simpler criteria for describing the magnetic properties of organic molecules for the study of aromaticity were sought. Dauben, Wilson and Laity proposed an elegant approach: similar to how thermodynamic quantities for molecules can be estimated from corresponding increments for the bonds contained, magnetic susceptibilities can be traced back to bond-specific contributions. In the case of aromatic compounds, it is found that the measured susceptibilities are higher than the values estimated from increments. This effect is attributed to the special magnetic effects caused by the cyclic delocalization. The term magnetic susceptibility exaltation was introduced for the effect according to the equation
- Λ = χ M (measured) - χ M (increment)
For aromatic molecules, Λ >> 0 applies, non-aromatic molecules show Λ ~ 0. Since antiaromatic molecules try to minimize cyclic delocalization by changing the geometry, no significantly negative Λ values are determined (cf. cyclooctatetraene).
Based on the values for "classic" aromatics listed in the adjacent figure, it can be deduced that these are characterized by Λ> 12 and that the size of Λ correlates with the size of the π systems. The series Cyclooctane → Cyclooctatetraen shows that the simple introduction of double bonds and their non-cyclic conjugation only have a negligible influence on the size of Λ. Finally, cyclooctatetraene largely evades the energetically unfavorable antiaromatic state through geometric distortion, so that although a negative value is determined for Λ, it is surprisingly low at −0.9. Interesting special cases are encountered with cyclopentadiene and 5,5-dimethylcyclopentadiene as well as with cycloheptatriene. The strikingly high values of the increase in magnetic susceptibility of the cyclopentadienes were interpreted as an indication of aromatic character through the inclusion of two electrons from the CH / CC bonds of the methylene unit; the high Λ value of cycloheptatriene is consistent with the effect of homoaromaticity.
The consideration of magnetic susceptibilities has experienced a temporary renaissance through the introduction of powerful quantum mechanical methods. Cremer et al. calculated magnetic susceptibilities to demonstrate the homoaromatic nature of the homotropylium cation. When the C1-C7 bond length is varied, the cation shows the highest magnetic susceptibility in its equilibrium geometry. The effect goes hand in hand with the greatest difference in the chemical shifts of H8-endo and H8-exo, the greatest alignment of the C1 – C7 13 C resonances and the strongest bond length compensation between the carbon atoms involved in the cyclic delocalization. These studies were extended to include a bishomoaromatic 1,4-bishomotropylium cation.
Herges and Schleyer used quantum-chemically calculated magnetic susceptibilities and their anisotropies to determine the aromatic character of transition states of pericyclic reactions such as B. to detect the Diels-Alder reaction . The calculation of magnetic susceptibilities and their anisotropies has now been replaced by other methods such as the calculation of NICS values or the visualization and quantification of induced ring currents, which give a more detailed insight into the phenomenon of aromaticity.
Geometric effects
As a characteristic feature of cyclic electron delocalization - starting from the "classic" case of π-aromaticity - the bond length compensation between single and double bonds is emphasized. Benzene, as the archetype of aromatics, shows this phenomenon in perfection, as does borazine , which can at best be seen as a borderline case for aromaticity (strong localization of electrons on the nitrogen atoms, low ring current). Schleyer et al. point out that the bond length differences in polycyclic aromatics do not have to differ significantly from those of linear conjugated polyenes and may even be smaller in the linear systems (see figure). Bond length compensation is therefore not in itself a sufficient criterion for aromatic character.
Individual evidence
- ↑ a b c d e P. von Ragué-Schleyer, Haijun Jiao: What is aromaticity ?. In: Pure and Applied Chemistry. 68, 1996, p. 209, doi: 10.1351 / pac199668020209 .
- ↑ Entry on aromaticity . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.A00442 Version: 2.3.3.
- ↑ a b c d e Hyp J. Dauben, James Dennis. Wilson, John L. Laity: Diamagnetic susceptibility exaltation as a criterion of aromaticity. In: Journal of the American Chemical Society. 90, 1968, p. 811, doi: 10.1021 / ja01005a059 .
- ↑ a b Michael JS Dewar: Chemical implications of σ conjugation. In: Journal of the American Chemical Society. 106, 1984, p. 669, doi: 10.1021 / ja00315a036 .
- ↑ Dieter Cremer: Pros and cons of σ-aromaticity. In: Tetrahedron. 44, 1988, p. 7427, doi : 10.1016 / S0040-4020 (01) 86238-4 .
- ^ Z. Chen, RB King: Spherical Aromaticity: Recent Work on Fullerenes, Polyhedral Boranes, and Related Structures . In: Chem. Rev. , 2005 , 105 , pp. 3613-3642.
- ↑ An overview of the current discussion on the subject of aromaticity and antiaromaticity can be found in Chemical Reviews Vol. 101, Issue 5, 2001 and Vol. 105, Issue 10, 2005.
- ↑ a b c d e f g h i j Z. Chen, CS Wannere, C. Corminboeuf, R. Puchta, P. v. R. Schleyer: Nucleus-Independent Chemical Shifts (NICS) as an Aromaticity Criterion . In: Chemical Reviews , 2005, 105, p. 3842, doi : 10.1021 / cr030088 +
- ↑ A. Kekulé: Sur la constitution des substances aromatique . In: Bull. Soc. Chim. Paris , 1865 , 3 , pp. 98-111. pdf
- ↑ E. Erlenmeyer: Studies on the so-called aromatic acids . In: Ann. , 1866 , 137 , pp. 327-359.
- ↑ P. Pascal: Recherches Magnétochimiques . In: Ann. Chim. Phys. , 1910 , 19 (8), pp. 5-70. pdf
- ↑ JW Armit, R. Robinson: CCXI. — Polynuclear heterocyclic aromatic types. Part II. Some anhydronium bases . In: J. Chem. Soc., Trans. , 1925 , 127 , pp. 1604-1618.
- ^ E. Hückel: Quantum theoretical contributions to the benzene problem . In: Z. Physik , 1931 , 70 , 204-286 and 72 , pp. 310-337.
- ^ L. Pauling: The Diamagnetic Anisotropy of Aromatic Molecules . In: J. Chem. Phys. , 1936 , 4 , pp. 673-677.
- ^ F. London: Théorie quantique des courants interatomiques dans les combinaisons aromatiques . In: J. Phys. Radium 1937 , 8 , pp. 397-409.
- ↑ JA Pople: Proton Magnetic Resonance of Hydrocarbons . In: J. Chem. Phys. , 1956 , 24 , p. 1111.
- ↑ RC Benson, WH Flygare: Molecular Zeeman effect of cyclopentadiene and isoprene and comparison of the magnetic susceptibility anisotropies . In: J. Am. Chem. Soc. , 1970 , 92 , pp. 7523-7529.
- ^ J. Aihara: Three-dimensional aromaticity of polyhedral boranes . In: J. Am. Chem. Soc. , 1978 , 100 , pp. 3339-3342.
- ^ W. Kutzelnigg: Theory of Magnetic Susceptibilities and NMR Chemical Shifts in Terms of Localized Quantities . In: Isr. J. Chem. , 1980 , 19 , pp. 193-200.
- ↑ P. v. Ragué-Schleyer, C. Maerker, A. Dransfeld, H. Jiao, NJR v. E. Hommes: Nucleus-Independent Chemical Shifts: A Simple and Efficient Aromaticity Probe . In: J. Am. Chem. Soc. , 1996 , 118 , pp. 6317-6318.
- ↑ K. Freitag, C. Gemel, P. Jarabek, IM Oppel, RW Seidel, G. Frenking, H. Banh, K. Dilchert, RA Fischer: The σ-Aromatic Clusters [Zn 3 ] + and [Zn 2 Cu] : Embryonic Brass . In: Angew. Chem. Int. Ed. , 2015 , 54 , pp. 4370-4374.
- ^ RV Williams: Homoaromaticity . In: Chem. Rev. , 2001 , 101 , pp. 1185-1204.
- ^ Z. Chen, RB King: Spherical Aromaticity: Recent Work on Fullerenes, Polyhedral Boranes, and Related Structures . In: Chem. Rev. , 2005 , 105 , pp. 3613-3642.
- ^ JD Roberts, MC Caserio: Basic Principles of Organic Chemistry , WA Benjamin Inc., New York, Amsterdam, 1965 .
- ^ Alan R. Katritzky, Karl Jug, Daniela C. Oniciu: Quantitative Measures of Aromaticity for Mono-, Bi-, and Tricyclic Penta- and Hexaatomic Heteroaromatic Ring Systems and Their Interrelationships . In: Chem. Rev. , 2001 101 , pp. 1421-1449, doi: 10.1021 / cr990327m and literature cited there.
- ↑ a b Michał Ksawery Cyrański: Energetic Aspects of Cyclic Pi-Electron Delocalization: Evaluation of the Methods of Estimating Aromatic Stabilization Energies . In: Chem. Rev. 2005 , 105 , pp. 3773-3811, doi: 10.1021 / cr0300845 .
- ↑ Entry on homodesmotic reaction . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.HT07048 Version: 2.3.3.
- ↑ The term "homodesmotic" is not always used uniformly in the literature. A critical commentary and a proposal for standardization can be found in SE Wheeler, KN Houk, P. v. R. Schleyer, WD Allen: A Hierarchy of Homodesmotic Reactions for Thermochemistry . In: J. Am. Chem. Soc. , 2009 , 131 , pp. 2547-2560.
- ↑ Schleyer et al. in Chem. Rev. , 2005 , 105 , pp. 3842-3888 and literature cited there.
- ↑ The reaction used is described by the authors as homodesmotic , but does not meet the definition of the term, cf. the discussion by Cyrański in Chem. Rev. , 2005 , 105 , pp. 3773-3811.
- ↑ P. v. Ragué-Schleyer, P. Freeman, H. Jiao, B. Goldfuss: Aromaticity and Antiaromaticity in Five-Membered C 4 H 4 X Ring Systems: “Classical” and “Magnetic” Concepts May Not Be “Orthogonal” . In: Angew. Chem. Int. Ed. , 1995 , 34 , pp. 337-340.
- ↑ Cyclopentadiene is discussed by the authors as a borderline case of an aromatic system, where the cyclic delocalization is achieved by including two electrons from the CH2 group. See also the literature cited in the article.
- ↑ Kenneth B. Wiberg: Antiaromaticity in Monocyclic Conjugated Carbon Rings. In: Chemical Reviews. 101, 2001, p. 1317, doi: 10.1021 / cr990367q .
- ↑ Modern quantum chemical calculation methods make it possible to calculate the magnetic properties of molecules today. Such methods were not available when the criterion of diamagnetic anisotropy was introduced to study aromatic compounds.
- ^ A b D. Cremer, P. Svensson, E. Kraka, Z. Konkoli, P. Ahlberg: Exploration of the Potential Energy Surface of C 9 H 9 + by ab Initio Methods. 2. Is the 1,4-Bishomotropylium Cation a Bishomoaromatic Prototype? . In: J. Am. Chem. Soc. , 1993 , 115 , pp. 7457-7464.
- ^ D. Cremer, F. Reichel, E. Kraka: Homotropenylium Cation: Structure, Stability, Magnetic Properties . In: J. Am. Chem. Soc. , 1991 , 113 , pp. 9459-9446.
- ↑ R. Herges, H. Jiao, P. v. R. Schleyer: Magnetic Properties of Aromatic Transition States: The Diels-Alder Reactions . In: Angew. Chem. Int. Ed. Engl. , 1994 , 33 , pp. 1376-1378.