Azithromycin
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
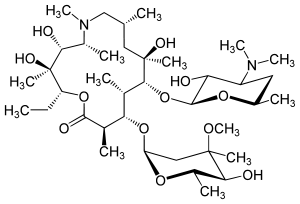
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Azithromycin | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 38 H 72 N 2 O 12 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 748.98 g · mol -1 | ||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Azithromycin is an organic chemical compound which, as a drug with an antibiotic effect, is one of the macrolide antibiotics . Since the macrocyclic lactone ( macrolide ) present as a glycoside contains an N atom in the large ring , azithromycin belongs to the group of azalides .
history
A research group at the pharmaceutical company Pliva in Zagreb in what was then Yugoslavia around Slobodan Dokić, Gabrijela Kobrehel, Gorjana Radobolja-Lazarevski and Zrinka Tamburašev discovered azithromycin in 1979/1980. It was patented in 1981 and was available from 1988 as Sumamed in the area of socialist Central and Eastern Europe. A contract was signed with Pfizer as early as 1986, which enabled Pfizer to be launched in Western Europe and outside Europe under the trade name Zithromax in 1991 .
application
Azithromycin is used for infections of the respiratory tract including pneumonia , acute exacerbation of chronic bronchitis , sinusitis , sore throat and tonsillitis . Azithromycin is also used for acute otitis media , skin and wound infections, Lyme borreliosis , bacterial conjunctivitis , for urethritis caused by chlamydia and for the prophylaxis of so-called MAK infection (Mycobacterium avium intracellular complex infection) in immunocompromised patients.
In dogs, the active ingredient has good effectiveness against canine papillomatosis .
Mechanism of action
Macrolide antibiotics hinder the process of protein synthesis during the lengthening phase of the protein chain on the ribosome by binding to the 50S subunit of the bacterial ribosome. By binding, they block the translocation , i.e. the displacement of the peptidyl-t- RNA from the acceptor site to the donor site. This leads to a premature interruption of protein synthesis and thus to a bacteriostatic effect. Azithromycin works slightly worse against gram-positive bacteria, but slightly better against gram-negative bacteria than other macrolides . What is special about this active ingredient is that it remains in the affected tissues for a long time, i.e. throat, pharynx and airways. It is also strongly enriched in the body's own immune cells, but only very slowly broken down. The advantage of this property: The drug only needs to be taken by the patient for three days, but it continues to have an effect for up to four days due to the delayed breakdown. This reduces the negative effect on the digestive system. The disadvantage is that it remains in the body for a long time in insufficient concentration. This promotes the development of resistance , since the growth of germs is no longer inhibited, but the pathogens are still exposed to the substance.
unwanted effects
Common (less than 10% of patients): gastrointestinal disorders such as loss of appetite, nausea, vomiting, diarrhea, loose stools, abdominal pain and cramps, indigestion and constipation.
Uncommon (less than 1% of patients): flatulence, taste disorders, fungal infections, vaginal infections, allergic reactions with rash, itching and nettle rash, nervousness, drowsiness, drowsiness, headache, abnormal sensations (paresthesia) and tiredness.
Rare (less than 0.1% of patients): dizziness, convulsions, seizures, hyperactivity, aggressive reactions, malaise, weakness, excitement, anxiety, low blood pressure, palpitations, cardiac arrhythmias, severe persistent diarrhea, photosensitivity reactions (skin reactions in connection with sunlight), Joint pain and tongue discoloration. Serious allergic reactions have been observed very rarely.
Therapy with azithromycin can lead to an increase in transaminases . Azithromycin is therefore contraindicated in severe liver function damage. Another contraindication are known allergic reactions to azithromycin or other antibiotics from the group of macrolides.
Azithromycin has an ototoxic potential. For this reason, tinnitus occurs in rare cases . The resulting damage to the inner ear should be reversible.
In an American study it was found that the risk of cardiac death increased slightly during a 5-day therapy with azithromycin. One additional cardiovascular death per 21,000 treated patients ( number needed to harm : 1: 21,000) was observed, especially in patients who had previously suffered from cardiovascular diseases. However, this must be weighed against treatment that may be more effective than with other antibiotics. However, another large Danish nationwide cohort study could not show an increased risk of mortality in patients under 65 years of age , so that administration in young patients with healthy heart does not represent an increased risk.
See also
Trade names
Azyter (D), InfectoAzit (D), Ultreon (D), Zithromax (D, A, CH), Azi-Teva (D), Sumamed (HR, PL), numerous generics (D, A, CH) Azitromicina (Costa Rica, Columbia, Peru, Ecuador, Dominican Republic)
Web links
Individual evidence
- ↑ a b Azithromycin data sheet from Sigma-Aldrich , accessed on October 17, 2016 ( PDF ).
- ↑ AZITHROMYCIN CRS data sheet (PDF) at EDQM , accessed on August 3, 2008.
- ↑ Z. Banić Tomišić: The Story of azithromycin . Kemija u industriji, year 2011, volume 60, issue 12, pp. 603–617; The Story of Azithromycin ( Memento from September 8, 2017 in the Internet Archive )
- ↑ BB Yagci et al .: Azithromycin therapy of papillomatosis in dogs: a prospective, randomized, double-blinded, placebo-controlled clinical trial. In: Vet. Dermatol. 19 (2008), pp. 194-198.
- ↑ D. Schneider, F. Richling: Checklist Medicines A – Z. 2006-2007. 4th edition. Thieme, Stuttgart 2006. p. 180. ISBN 3-13-130854-0 .
- ↑ Ray, WA. et al. (2012): Azithromycin and the risk of cardiovascular death . In: N Engl J Med 366 (20); 1881-1890; PMID 22591294 .
- ↑ Andrew D. Mosholder, Justin Mathew, John J. Alexander, Harry Smith, Sumathi Nambiar: Cardiovascular Risks with Azithromycin and Other Antibacterial Drugs New England Journal of Medicine 2013; Volume 368, Issue 18 of May 2, 2013, pp. 1665-1668; doi: 10.1056 / NEJMp1302726 .
- ↑ Henrik Svanström, Björn Pasternak, Anders Hviid: Use of Azithromycin and Death from Cardiovascular Causes New England Journal of Medicine 2013; Volume 368, Issue 18 of May 2, 2013, pp. 1704-1712; doi: 10.1056 / NEJMoa1300799 .