Carbamazepine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
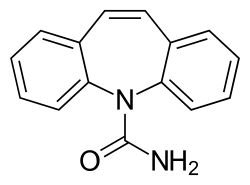
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Carbamazepine | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 15 H 12 N 2 O | ||||||||||||||||||
| Brief description |
white to almost white, crystalline and polymorphic powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| Mechanism of action |
Blockage of the sodium channels |
||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 236.27 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
|
||||||||||||||||||
| solubility |
very sparingly soluble in water (205 mg l −1 ), slightly soluble in dichloromethane , slightly soluble in acetone and ethanol 96% |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Chemically, carbamazepine belongs to the class of dibenzazepines and is an anticonvulsant that is mainly used against focal epilepsies . In addition, it is also used as a phase prophylactic for various psychiatric illnesses. In terms of its structure, it is similar to imipramine .
presentation
The synthesis starts with 2-nitrotoluene (1), which is reacted radically with chlorine ( SSS rule ). A strong base generates the corresponding carbanion from (2) at the CH-acidic site . Another molecule of 2-nitrobenzyl chloride reacts with (3) via a nucleophilic substitution ( S N 2 mechanism) to form (4). A second order elimination reaction gives (5). A hydrogenation using a palladium / carbon catalyst is produced (6), which reacts after boiling to a hydrogenated dibenzoazepine (7). With N-bromosuccinimide is simply brominated and then re-made on a second-order elimination mechanism Dibenzoazepin (9). By means of a nucleophilic substitution , phosgene reacts with the nitrogen nucleophile to form carbamic acid chloride and then with ammonia to form carbamazepine (11).
Analytics
The coupling of HPLC with mass spectrometry after appropriate sample preparation is suitable for the reliable qualitative and quantitative determination of carbamazepine in various test items.
pharmacology
Pharmacokinetics
Carbamazepine is absorbed relatively slowly (2–8 hours) and has a bioavailability of around 80%. The metabolic by-product carbamazepine-10,11-epoxide also has anti-epileptic properties, but is also considered to be responsible for the toxic effects of the substance. The therapeutic range is narrow .
Carbamazepine is processed in the liver via the cytochrome P450 enzyme system (mainly CYP3A4 , but also 1A2 and 2C9 ), the activity of which it also induces . This is particularly relevant with regard to potential (drug) interactions .
Pharmacodynamics (mechanism of action)
The mechanism of action is not yet fully understood. However, it is assumed that carbamazepine acts by blocking (voltage-dependent) sodium channels in the axons of the nerve cells and thus on the (ectopic) spread of excitation and has a membrane-stabilizing effect.
Clinical information
Possible indications
In addition to treating epilepsies , another important indication for carbamazepine is for treating mood disorders such as mania . It is indicated above all for the acute treatment of mania and schizomaniac episodes as well as for phase prophylaxis of bipolar and schizoaffective disorders, but is increasingly being replaced by more modern preparations. In addition, carbamazepine is used to protect against withdrawal attacks in benzodiazepine and alcohol withdrawal . Carbamazepine is also used to treat trigeminal neuralgia .
It can also be used in the treatment of borderline personality disorder in the case of severe mood swings and autoaggression . Furthermore, its use as a coanalgesic in neuropathic pain plays an important role.
Use during pregnancy and breastfeeding
Carbamazepine can cause malformations in the unborn child ( teratogenic effect). In particular, the risk of gaps in the spine ( spina bifida , "open back") is increased. More recent studies, however, indicate only a slight increase in the incidence of major malformations. The combination with other anticonvulsants can further increase the malformation rate. For treatment with carbamazepine during pregnancy, the risk-benefit assessment must be made particularly carefully.
Side effects
Possible side effects occur predominantly in a dose-dependent manner, especially at the start of treatment, and disappear after a few days, if necessary after a temporary dose reduction or by themselves. Very often there is a depressant effect on the central nervous system up to drowsiness, balance disorders and temporary benign reductions in the number of white blood cells. Other changes in the blood count, including a decrease in the number of platelets (thrombopenia), may often occur. Also common are allergic skin reactions, loss of appetite, dry mouth, nausea, vomiting and a disorder of the salt balance with reduced sodium content in the blood. Occasionally, water retention can also lead to weight gain. Also occasionally headaches, states of confusion, especially in elderly patients, movement disorders with involuntary movements or eye movement disorders and slow heart rate as well as other cardiac arrhythmias are observed. Water intoxication with accompanying symptoms, diarrhea, but also constipation and jaundice or an acute allergic liver inflammation occur only rarely. The very rare but possibly life-threatening side effects include agranulocytosis , but also psychiatric symptoms such as depressive or manic moods, anxiety disorders, aggressive behavior disorders, hallucinations and activation of latent psychoses . If changes in the blood count or allergic rashes occur, the substance must be discontinued. In rare cases, drug-toxic alveolitis ( IPF ) can also occur with carbamazepine , which can regress on its own if you are not given any further therapy. The substance was considered a frequent trigger of the DRESS syndrome .
In people with certain gene variations in the HLA system , hypersensitivity reactions can occur from maculopapular rash to severe liver and kidney diseases to Stevens-Johnson syndrome and toxic epidermal necrolysis . On the one hand, there are people of Asian origin with the genotype HLA-B * 1502 (frequency 2/1000), this genotype is found even more rarely in the Central and Northern European population, in 2011 the genotype HLA-A * 3101 (frequency 2 –5%) identified as a trigger. The genotyping before prescribing may be useful, the FDA writes before.
Interactions
By activating cytochrome P450 isoenzymes in the liver, carbamazepine accelerates the breakdown of other drugs in addition to its own, such as phenprocoumon, birth control pills , some antidepressants and neuroleptics , cyclosporine , astemizole , valproic acid and many more Prescribed drugs lose their effect when carbamazepine is administered, so a check of the serum level and a dose adjustment is necessary.
Special attention should be paid to drugs which, on the other hand, inhibit the metabolism of carbamazepine (increase in serum levels, risk of poisoning).
Substances that can increase the plasma concentrations of carbamazepine are:
- Grapefruit juice ,
- Fluoxetine ,
- Fluvoxamine ,
- possibly desipramine ,
- Isoniazid ,
- Verapamil ,
- Diltiazem ,
- Dextropropoxyphene,
- Viloxazin ,
- possibly cimetidine ,
- Acetazolamide ,
- Danazol ,
- Nicotinamide (in adults and only in high doses),
- Nefazodone , macrolide antibiotics (e.g. erythromycin , troleandromycin, josamycin, clarithromycin ),
- Azole derivatives (e.g. itraconazole, ketoconazole , fluconazole ),
- Terfenadine ,
- Loratadine ,
- Protease inhibitors used to treat HIV
Substances that can lower the plasma concentration of carbamazepine are, however:
- herbal preparations containing St. John's wort (Hypericum perforatum)
- Phenobarbital ,
- Primidones ,
- Progabide,
- Theophylline ,
- Mesuximide ,
- Rifampicin ,
- Cisplatin ,
- Doxorubicin ,
- Clonazepam ,
- Valproic acid or valpromide,
- Oxcarbazepine
Effects on ability to drive or use machines
The patient's ability to react quickly may be affected by dizziness or sleepiness, especially at the beginning of therapy or in connection with dose adjustments.
Contraindications
In the presence of conduction disorders of the heart, carbamazepine is contraindicated.
dosage
Carbamazepine should initially be increased slowly in the dose ( creeping in ), since side effects occur especially at the beginning of therapy. The target serum level is 6 to 8 (12) μg / ml. Are particularly suitable for slow-release forms . During the course of therapy, regular dose adjustments (activation of the liver metabolism) and monthly check-ups (side effects) are necessary. At the end of the therapy, the dose must be gradually reduced.
Environmental impact
According to the Austrian Federal Environment Agency, the pharmaceutical residues regularly found in sewage and sewage sludge are often carbamazepine.
literature
- Stefan Brunnhuber, Sabine Frauenknecht, Klaus Lieb: Intensive course in psychiatry and psychotherapy. Elsevier, Urban & Fischer, Munich 2005, ISBN 3-437-42131-X , pp. 60f.
- Günter Krämer , Hans-Christian Hopf, editor. Carbamazepine in neurology . G. Thieme, Stuttgart - New York 1987
Trade names
Carbaflux (D), Carbagamma (D), Carsol (CH), Deleptin (A), Finlepsin (D), Neurotop (A, CH), Tegretal (D), Tegretol (A, CH), Timonil (D, CH) , numerous generics (D, A)
Web links
Individual evidence
- ↑ a b European Pharmacopoeia Commission (Ed.): EUROPEAN PHARMACOPOE 6TH EDITION . tape 6.0-6.2 , 2008.
- ↑ a b Jia Deng, Sven Staufenbiel, Roland Bodmeier: Evaluation of a biphasic in vitro dissolution test for estimating the bioavailability of carbamazepine polymorphic forms in Eur. J. Pharm. Sci. 105 (2017) 64-70, doi: 10.1016 / j.ejps.2017.05.013 .
- ↑ K. Tsinman, A. Avdeef, O. Tsinman, D. Voloboy: Powder Dissolution Method for Estimating Rotating Disk Instrinsic Dissolution Rates of Low Solubility Drugs. In: Pharm Res . 26, 2009, pp. 2093-2100. doi: 10.1007 / s11095-009-9921-3 .
- ↑ Entry on carbamazepine in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ↑ a b Data sheet carbamazepine from Sigma-Aldrich , accessed on March 15, 2011 ( PDF ).
- ↑ Rivera-Jaimes JA, Postigo C, Melgoza-Alemán RM, Aceña J, Barceló D, López de Alda M: Study of pharmaceuticals in surface and wastewater from Cuernavaca, Morelos, Mexico: Occurrence and environmental risk assessment. , Sci Total Environ. 2018 Feb 1; 613-614: 1263-1274, PMID 28962074
- ↑ Meyer W, Reich M, Beier S, Behrendt J, Gulyas H, Otterpohl R: Measured and predicted environmental concentrations of carbamazepine, diclofenac, and metoprolol in small and medium rivers in northern Germany. , Environ Monit Assess. 2016 Aug; 188 (8): 487, PMID 27465046
- ↑ El Hamd MA, Wada M, Ikeda R, Kawakami S, Nakashima K: Validation of an LC-MS / MS Method for the Determination of Propofol, Midazolam, and Carbamazepine in Rat Plasma: Application to Monitor Their Concentrations Following Co-administration. , Biol Pharm Bull. 2015; 38 (8): 1250-3, PMID 26235591
- ↑ Torsten Kratz, Albert Diefenbacher: Psychopharmacotherapy in old age. Avoidance of drug interactions and polypharmacy. In: Deutsches Ärzteblatt. Volume 116, Issue 29 f. (July 22) 2019, pp. 508-517, pp. 509-511.
- ↑ Richard Daikeler, idols Use, Sylke Waibel: diabetes. Evidence-based diagnosis and therapy. 10th edition. Kitteltaschenbuch, Sinsheim 2015, ISBN 978-3-00-050903-2 , p. 173.
- ↑ Guideline trigeminal neuralgia of the German Society for Neurology . In: AWMF online (as of 02/2005)
- ↑ Guidelines of the German Society for Child and Adolescent Psychiatry and Psychotherapy. (old) ( Memento from July 12, 2010 in the web archive archive.today )
- ↑ a b Embriotox drug safety during pregnancy and lactation: data for carbamazepine. ( Memento of June 26, 2010 in the Internet Archive ) Retrieved March 7, 2012.
- ↑ Technical information Tegretal 600 mg retard, as of September 2011.
- ↑ a b c d e Specialist information Carbamazepine-ratiopharm, as of January 2013.
- ^ GG King, DJ Barnes, MJ Hayes: Carbamazepine-induced pneumonitis . In: The Medical Journal of Australia . tape 160 , no. 3 , February 1994, p. 126-127 , PMID 8295578 .
- ↑ Carbamazepine: genetic test predicts severe skin reactions. (No longer available online.) Aerzteblatt.de, March 24, 2011, archived from the original on September 1, 2014 ; Retrieved July 1, 2012 .
- ↑ Carbamazepine hypersensitivity can be predicted by genetic testing, Der Arzneimittelbrief, Volume 45, June 2011.
- ↑ a b Technical information of the Swiss Medicines Compendium: Carsol® CR; Information as of January 2004.
- ^ Specialist information from the Swiss Medicines Compendium: Tegretol, as of September 2002.
- ↑ Richard Daikeler, idols Use, Sylke Waibel: diabetes. Evidence-based diagnosis and therapy. 10th edition. Kitteltaschenbuch, Sinsheim 2015, ISBN 978-3-00-050903-2 , p. 173.
- ↑ Federal Environment Agency: Carbamazepine and caffeine - potential screening parameters for municipal groundwater contamination? . Vienna, 2006.
