Characteristic X-rays
The characteristic X-ray radiation is a line spectrum of X-ray radiation that arises at transitions between energy levels of the inner electron shell and is characteristic of the respective element . It was discovered by Charles Glover Barkla , who received the Nobel Prize in Physics for it in 1917 .
Emergence
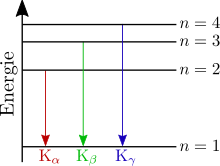
The characteristic lines of the X-ray spectrum ( , , ...) created in the image of shell model as follows:
- One of the free, high-energy electrons of the electron beam knocks out an electron that is bound according to the electron configuration in the inner shell of its atom. In doing so, at least as much energy must be transferred to the impacted electron as is necessary to jump onto a still unoccupied shell. Usually the impact energy is larger than the previous binding energy of the electron and the atom is ionized .
- The resulting gap is closed by an electron from a shell further out. To do this, the higher-energy electron of the shell located further outside has to give up the difference in energy when changing to a shell located further inside. It emits a photon (radiation quantum).
The photon energy is typically in the order of magnitude of 1-100 keV corresponding to the energy difference of the electron shell in the two states (missing electron in the inner shell and in the outer shell) and is therefore in the electromagnetic spectrum in the X-ray range. The radiation quanta therefore have the energy difference between the higher (e.g. L-) and lower (e.g. K-) shell. Since this energy difference is element-specific, this X-ray radiation is called characteristic X-ray radiation .
The wavelength and thus the energy of the emitted radiation can be calculated using Moseley's law .
Designation of the spectral lines
To designate the X-ray lines, first specify the inner shell into which the electron went during the emission, e.g. B. K, L, M etc. A Greek letter as an index indicates the difference to the principal quantum number n of the outer shell from which the electron came. E.g. corresponds to
- an index one of 1, i.e. H. the next higher shell (for the K series this is the L shell)
- an index one of 2 (for the K series this is the M shell) etc.
With the L and M series as well as with atoms with a higher atomic number , this assignment is no longer so clear. The breakdown of the fine structure plays a role here. In addition to the Greek index, a numerical index is used to distinguish the lines.
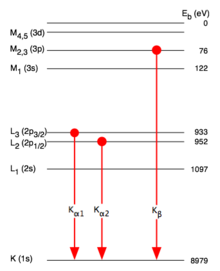
Appearance of several spectral lines after an electron excitation
Atoms with a higher atomic number have several outer shells that can supply an electron to fill the hole in the inner shell. The hole can also arise in different inner shells. Accordingly, these atoms can also emit X-rays of different energies.
- After an electron z. B. has fallen from the L to the K shell, the L shell is again understaffed. Another electron from an even higher shell falls down, emitting another photon . This second photon is of lower energy and contributes to the L-line in this example.
- In addition to the X-ray emission - especially in the case of light atoms with atomic numbers - the transfer of energy to electrons further out is another way of compensating for the energy difference (see Auger effect ).
Generation in the X-ray tube

In an X-ray tube , high-energy electrons hit an anode and there generate both characteristic X-rays and bremsstrahlung . In the graphically represented spectrum, the lines of the characteristic X-ray radiation appear as high elevations ( peaks ) on the continuous background of the bremsstrahlung.
application
The characteristic X-ray radiation is observed with detectors that determine the energy or the wavelength of the X-ray quanta. From the spectrum, qualitative conclusions can be drawn about the elemental composition of the sample; a ZAF correction also enables a quantitative analysis. This principle is used in X-ray fluorescence analysis , energy-dispersive (EDX / EDS) and wavelength-dispersive X-ray spectroscopy (WDX / WDS).
Web links
- Database (X-Ray Transition Energies Database) for the energies of the characteristic X-rays (theoretical and experimental) of various substances
- LP: Characteristic Radiation , Georg-August-Universität Göttingen. Notes in particular on the notation.