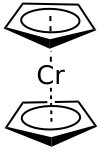
Chromocene
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| Ecliptic conformation | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Chromocene | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 10 H 10 Cr | |||||||||||||||
| Brief description |
red crystalline solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 182.18 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.43 g cm −3 |
|||||||||||||||
| Melting point |
173 ° C |
|||||||||||||||
| solubility |
almost insoluble |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Chromocene , with the formula [Cr (C 5 H 5 ) 2 ], is an organometallic compound from the family of metallocenes . The empirical formula is often abbreviated as [Cr (Cp) 2 ]. It forms a sandwich complex analogous to ferrocene , but does not follow the 18-electron rule because it has only 16 valence electrons . Chromocene was first described in 1953.
Manufacturing
Chromocene is made by reacting chromium (II) chloride with cyclopentadienyl sodium , usually in tetrahydrofuran (THF) as a solvent. In the first step, the anhydrous chromium (II) chloride is first synthesized:
Direct production from chromium (II) chloride, cyclopentadiene and sodium is also possible:
The analogous decamethylchromocene, Cr [C 5 (CH 3 ) 5 ] 2 , is prepared analogously to the first reaction using pentamethylcyclopentadienyllithium, LiC 5 (CH 3 ) 5 .
properties
Chromocene is a red crystalline solid. In the crystal structure of chromocene, the mean Cr – C bond length was determined to be 215.1 (13) pm by means of X-ray structure analysis. As in ferrocene, the cyclopentadienyl rings in chromocene are arranged in congruence ( ecliptic ) and not staggered. The dissociation energy for the Cp – Cr bond is given as 179 kJ · mol −1 or 279 kJ · mol −1 , depending on the literature . The mean distance between the rings is 370 pm. Chromocene is very reactive in air and towards water and can under certain circumstances ignite spontaneously on contact with air.
use
Chromocene applied to silicate carrier substances acts as a catalyst in the polymerization of ethylene and other 1-alkenes. Union Carbide developed this process for the polymerization of ethylene in the 1960s . The chromocene decomposes on the silicate surface with the formation of highly reactive organometallic centers, which result in the catalytic effectiveness. The structure and mode of action of the Union Carbide catalyst has been studied in detail by Janet Blümel. Chromocene-based catalysts have the advantage over Ziegler catalysts which contain organoaluminum compounds that the polyethylene produced has only a low odor nuisance. Alternatively, aerated concrete can also be used as a substrate (carrier substance) .
Individual evidence
- ↑ a b c Christoph Elschenbroich: Organometallchemie . BG Teubner Verlag, 2008, ISBN 978-3-8351-0167-8 ( p. 452 in the Google book search).
- ↑ E. Weiss, EO Fischer: On the crystal structure of di-cyclopentadienyl-chromium (II) . In: Z. Anorg. General Chem. Band 284 , 1956, pp. 69-72 , doi : 10.1002 / zaac.19562840109 .
- ↑ Data sheet bis (cyclopentadienyl) chromium (PDF) from Strem, accessed on January 10, 2012.
- ↑ a b Data sheet bis (cyclopentadienyl) chromium (II) from Sigma-Aldrich , accessed on December 4, 2011 ( PDF ).
- ^ EO Fischer, W. Hafner: Di-cyclopentadienyl-chromium. In: Journal of Nature Research B . 8, 1953, pp. 444-445 ( online ).
- ↑ Chromocene (PDF, 117 kB)
- ↑ Patent EP0652224B1 : Process for the preparation of metallocenes in a one-step synthesis.
- ↑ Kevin R. Flower, Peter B. Hitchcock: Crystal and molecular structure of chromocene (η 5 -C 5 H 5 ) 2 Cr. In: Journal of Organometallic Chemistry . 1996, 507. pp. 275-277. doi : 10.1016 / 0022-328X (95) 05747-D .
- ^ A b c d A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , pp. 1699-1700.
- ↑ James E. Huheey, Ellen A. Keiter, Richard Keiter: Inorganic Chemistry: Principles of Structure and Reactivity . de Gruyter Verlag, 2003, ISBN 3-11-017903-2 ( p. 797 in the Google book search).
- ↑ F. Jellinek: The structure of the osmocene. In: Journal of Nature Research B . 14, 1959, pp. 737-738 ( online ).
- ↑ Patent DE4306105A1 : Modified supported chromocene catalyst systems.
- ^ Manfred Dieter Lechner, Klaus Gehrke, Eckhard H. Nordmeier: Macromolecular chemistry: a textbook for chemists, physicists . ( P. 93 in Google Book search).
- ↑ Ralf Alsfasser, Erwin Riedel, HJ Meyer: Moderne Anorganische Chemie . de Gruyter Verlag, ISBN 978-3-11-019060-1 ( page 708 in the Google book search).
- ↑ Dirk Steinborn: Fundamentals of organometallic complex catalysis . Vieweg + Teubner, 2007, ISBN 978-3-8348-0581-2 ( p. 158 in the Google book search).
- ↑ Catalyst-carrier interactions: Structure and mode of action of the Union Carbide catalyst (chromocene / silica) (PDF, 207 kB)
- ↑ Patent EP1109859B1 : Low odor polyethylene blends.
- ↑ Patent DE10314369B4 : Production of polymerization catalyst, used in polymerization of olefins, including styrene, especially ethylene, uses porous cement concrete as support for application of metallocene derivative.

![{\ mathrm {Cr \ + \ 2 \ HCl \ {\ xrightarrow [{THF}] {}} \ CrCl_ {2} \ + \ H_ {2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/66672eecfbbeffb0ecf729ffc06aad46f09ab335)
![{\ mathrm {CrCl_ {2} \ + \ 2 \ Na (C_ {5} H_ {5}) \ {\ xrightarrow [{THF}] {}} \ Cr (C_ {5} H_ {5}) _ { 2} \ + \ 2 \ NaCl}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/e1f4ab5e6046ec42ccf01629eb267e824a2b00e7)
![{\ mathrm {CrCl_ {2} \ + \ 2 \ Na \ +2 \ C_ {5} H_ {6} \ {\ xrightarrow [{THF}] {}} \ Cr (C_ {5} H_ {5}) _ {2} \ + \ 2 \ NaCl + \ H_ {2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/dfca751dea67ade67bdbf3ae17c8cf4c05630189)
