Coniferyl alcohol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
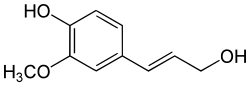
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Coniferyl alcohol | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 10 H 12 O 3 | ||||||||||||||||||
| Brief description |
colorless prisms |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 180.20 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
73-74 ° C |
||||||||||||||||||
| boiling point |
163-165 ° C (400 Pa ) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Coniferyl alcohol is a phenylpropanoid . It is the aglycon of coniferin and a component of softwood - lignin .
Extraction
Theodor Hartig was first able to characterize it in 1861 in the cambial sap of the larch. The Holzminden pharmacist Wilhelm Kubel identified the glucoside of coniferyl alcohol in 1866. It was first obtained in 1874 by F. Tiemann and W. Haarmann by acid hydrolysis of the glucoside coniferin or today by the enzyme emulsin (a β- glucosidase ).
biochemistry
Coniferyl alcohol is a metabolic intermediate in the biosynthesis of eugenol .
Individual evidence
- ↑ a b c d e f Entry on coniferyl alcohol. In: Römpp Online . Georg Thieme Verlag, accessed on December 28, 2014.
- ↑ Entry on coniferyl alcohol in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ Data sheet Coniferyl alcohol, 98% at AlfaAesar, accessed on February 9, 2019 ( PDF )(JavaScript required) .
- ↑ W. Kubel: Coniferin - a glucoside from the cambial sap of the conifers . In: Journal für Praktische Chemie 97 , 243-246 (1866). doi : 10.1002 / prac.18660970132 .
- ↑ F. Tiemann, W. Haarmann: About coniferin and its transformation into the aromatic principle of vanilla . In: Reports of the German Chemical Society 7 , 608–623 (1874). doi : 10.1002 / cber.187400701193 . Digitized on Gallica .