Cyanide
Cyanides are salts and other compounds of hydrocyanic acid ( hydrogen cyanide , HCN). In organic chemistry , “cyanide” is an outdated, but still very common term for nitriles - viewed as an ester of hydrogen cyanide - with the general formula R – C≡N. The name cyanide is derived from the Greek κυανός ( kyanos ) 'blue' and is derived from the extraction of iron hexacyanidoferrate ( Berlin blue ), a pigment with a blue color.
properties
The salt-like cyanides contain the cyanide anion [C≡N] - , the organic cyanides the functional group –C≡N. Water-soluble cyanides are partially hydrolyzed in moist air and smell like hydrocyanic acid .
Easy connections
All cyanides of the alkali and alkaline earth metals are highly toxic and easily soluble in water, such as potassium cyanide (cyanide) and sodium cyanide . The toxicity of these salts is due to the release of hydrocyanic acid when reacting with the hydrochloric acid of the stomach:
Potassium cyanide plays a role in electroplating .
Sodium cyanide is used to extract gold and silver .
Complex compounds (cyano compounds)
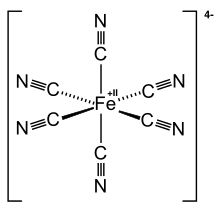
The cyanide anion is very reactive and forms very stable complex compounds with other metals (apart from alkali and alkaline earth metals) such as iron in particular. The cyanide ion has a monodentate character. Usually an anion is formed in which the metal forms the central atom of the cyanide building blocks. The metal forms the middle and is surrounded by the cyanide ions, as in [Fe (CN) 6 ] 4− . A type of connection has formed called a complex . With cations , salts such as K 4 [Fe (CN) 6 ], potassium hexacyanidoferrate (II) , the so-called yellow blood liquor salt, are formed. In many complex compounds, the cyanide is firmly bound so that its toxicity (reactivity) is lost. Some of the hydrocyanic acid can be released by adding hot, dilute sulfuric acid , so cyanide complexes must be handled with a certain degree of caution. Concentrated sulfuric acid does not release hydrocyanic acid, however, as it immediately hydrolyzes the hydrocyanic acid produced to carbon monoxide . Analytically, no cyanide can be detected in aqueous solutions of the complexes.
Use of complex compounds in the food industry
In the food industry, the cyanido complexes sodium ferrocyanide (E 535, sodium hexacyanidoferrate (II)), potassium ferrocyanide (E 536, potassium hexacyanidoferrate (II)) and calcium ferrocyanide (E 538, calcium hexacyanidoferrate (II)) are used as food additives . These salts are approved in small quantities as artificial flow aids , release agents and stabilizers for table salt and table salt substitutes.
Natural occurrence
Cyanides are found - bound in non-toxic cyanogenic glycosides - in the kernels of many fruits, for example in rose plants ( Prunus species such as plum ( Prunus domestica ), blackthorn ( Prunus spinosa ), apricot ( Prunus armeniaca ), almond ( Prunus dulcis ), peach ( Prunus persica ), sour cherry ( Prunus cerasus )), in Leguminosae (legumes), spurge as cassava ( Manihot esculenta ), grasses like sorghum , Leingewächsen , such as flax ( Linum usitatissimum ), Philodendron , composite flowers and passifloraceae , but also in ferns such as the golden spotted fern ( Phlebodium aureum ).
Poisonous effect
Cyanides such as hydrocyanic acid (HCN) and their alkali salts (e.g. KCN or NaCN) are highly toxic. Poisoning with these substances can occur in industrial and commercial areas as accidents, but also in the private sector as a result of intentional poisoning in the case of murder and suicide. The poison can either be absorbed through the lungs as gaseous hydrocyanic acid (e.g. by inhaling smoke containing cyanide), in liquid form (e.g. biting into a hydrocyanic acid capsule in the mouth), or orally as an alkali salt (e.g. Ingestion of potassium cyanide).
The mechanism of cyanide poisoning is based on the inhibition of the enzyme cytochrome c oxidase in the respiratory chain . Complexation of the Fe (III) ion takes place. This prevents the use of oxygen in the cell . Antidotes are 4-dimethylaminophenol hydrochloride (4-DMAP), sodium thiosulfate , hydroxycobalamin (vitamin B 12b , in the Cyanokit), and amyl nitrite . The binding of the cyanide to Fe (II) ions is comparatively low. The inactivation of hemoglobin by binding the Fe (II) ion therefore plays a subordinate role in poisoning.
The light red color of the skin is a typical sign of poisoning with cyanides: the venous blood is still enriched with oxygen because the cells could not use the oxygen.
Most cells have the enzyme rhodanase , which binds sulfur to the cyanide ion (CN - ), resulting in rhodanide (SCN - ). This detoxification in the body takes place at a rate of 0.1 mg per kg of body weight and hour. Therefore, a longer intake of smaller amounts of cyanide is harmless, symptoms of poisoning arise from the shock absorption.
Usually 4-DMAP ( 4-dimethylaminophenol ) or ethylenediaminetetraacetic acid cobalt (II) complexes are administered as antidotes.
proof
The high chemical detection of cyanides can with Fe 3+ - ions in hydrochloric acid solution by reaction with ammonium polysulfide done. This creates the deep red colored iron (III) thiocyanate Fe (SCN) 3 . However, it should be noted that this detection does not work in the presence of Fe (II) because of the formation of Prussian Blue , a complex of Fe (II) with hexacyanidoferrate (III) as ligand. However, the detection can also be carried out with a mixture of iron (II) and iron (III) salt, whereby Prussian blue is formed.
Practical use
Mining
In practice, cyanide solutions are used to extract precious metals from rocks ( gold and silver extraction ). This was first done in South Africa in the 1890s, using the MacArthur-Forrest method developed by John Stewart MacArthur in 1887. Due to the high toxicity of cyanide, this is associated with great potential damage to the environment. Environmental damage can result from the discharge of the sludge or from unsafe deposits. In 2000, in Baia-Mare , Romania , major environmental damage occurred as a result of a dam breach during gold processing; in Kütahya, Turkey, two out of three silver processing dams broke in 2011.
The European Parliament voted in May 2010 to ban the use of cyanide in mining. Environmental organizations criticize the fact that cyanide is still used in mining outside the EU. B. in the highlands of the Dominican Republic and Costa Rica , but also in the large mining nations of Australia, Canada and South Africa. According to a report from 2011, there were at least 30 major accidents involving cyanide in mining worldwide in the previous 25 years, often triggered by dam breaches. The cyanide is usually only used in low concentrations (typically 100 to 500 ppm in gold mining). Alternatives to the use of cyanide were developed, for example, in Australia (thiosulfate process of the CSIRO) and in 2017 by the Nobel Prize winner Fraser Stoddart (using hydrogen peroxide and corn starch).
Metalworking
When hardening metal, cyanide-containing hardening salts are used depending on the procedure. Used hardening salts containing cyanide then also contain alloy components of the workpieces to be hardened and are regularly hazardous waste (waste code 110301 * according to AVV ). If it is not possible to recycle waste , it can only be disposed of in underground landfills due to its high level of toxicity and water hazard.
disposal
To dispose of cyanides, use a suitable oxidizing agent such as sodium hypochlorite (NaOCl) or hydrogen peroxide (H 2 O 2 ). The cyanide is converted into harmless nitrogen and carbon dioxide .
Cyanide must never come into contact with acids during disposal, otherwise hydrogen cyanide is formed. The conversion for disposal must therefore take place in a basic environment. Hypochlorite anions must also not come into contact with acid, otherwise chlorine gas is released.
Even the autoprotolysis of the water ( ) is sufficient to donate the protons for the formation of the poisonous hydrocyanic acid . alkaline disposal must be strictly observed.
Web links
Individual evidence
- ↑ a b c Entry on cyanides. In: Römpp Online . Georg Thieme Verlag, accessed on March 25, 2013.
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 .
- ↑ Entry on cyanogenic glycosides. In: Römpp Online . Georg Thieme Verlag, accessed on April 27, 2012.
- ^ Franz-Xaver Reichl, Jochen Benecke: Pocket Atlas of Toxicology. P. 134.
- ↑ potassium cyanide. Retrieved February 6, 2020 .
- ↑ Ernst Mutschler (Ed.): Mutschler drug effects . 9., completely reworked and exp. Edition. Wissenschaftliche Verlagsgesellschaft, Stuttgart 2008, ISBN 978-3-8047-1952-1 .
- ↑ a b Jander-Blasius: Textbook of analytical and preparative inorganic chemistry. 8th edition, S. Hirzel Verlag, Stuttgart 1969.
- ↑ Nick Magel: Dam burst in Kütahya - environmental disaster in Turkey - cyanide liquor from silver mine leaked. In: TerraStormBochum. May 9, 2011, accessed August 10, 2012 .
- ↑ Die Presse.com: Environment: Cyanide gold mining over? , May 6, 2010.
- ↑ Deutschlandfunk: It's all chemistry that shines , December 29, 2009.
- ↑ regenwald.org: Toxic gold mining in the Caribbean , November 23, 2012.
- ↑ Heidi Vella, Should cyanide still be used in modern-day mining? , Mining Technology, March 7, 2016
- ^ Jon Yeomans, Nobel Prize winner Sir Fraser Stoddart hopes to turn gold mines green , The Telegraph, March 15, 2017
- ↑ on this and on disposal Waste profile "1103 Sludge and solids from hardening processes" , information portal on waste assessment (IPA) d. LANUV u. a. Waste authorities