D -luciferin
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | D-luciferin | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 11 H 8 N 2 O 3 S 2 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 280.32 g mol −1 | ||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
D- luciferin (also firefly luciferin ) is a chemical compound from the group of luciferins , which are used in bioluminescent organisms to generate light. In biochemistry , luciferin is used as a substrate for the reporter enzyme luciferase .
properties
In fireflies is D luciferin in the peroxisomes, are formed of the cells of the luminous body and oxidatively decarboxylated to generate light.
The D- luciferin is oxidatively decarboxylated to oxyluciferin (oxy-L) by the enzyme luciferase in the course of bioluminescence :
The reaction takes place in vitro at an optimum pH of 7.8 at room temperature. While the color of the bioluminescence in vivo is yellow-green to yellow (552-582 nm), the spectrum is larger in vitro . At an acidic pH there is a shift into the red spectrum (615 nm). It also absorbs light at 327 nm and emits fluorescence at 530 nm.
The biosynthesis of D - and L luciferin is carried out from two cysteines , and p-benzoquinone or hydroquinone . The chemical synthesis takes place analogously.
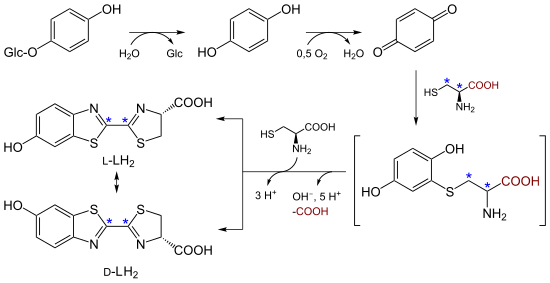
Applications
D- luciferin is used in conjunction with the use of luciferases as a reporter gene .
Individual evidence
- ↑ a b c data sheet D-Luciferin, from Sigma-Aldrich , accessed on December 18, 2017 ( PDF ).
- ↑ GA Keller, S. Gould, M. Deluca, S. Subramani: Firefly luciferase is targeted to peroxisomes in mammalian cells. In: Proceedings of the National Academy of Sciences of the United States of America . Volume 84, Number 10, May 1987, pp. 3264-3268, PMID 3554235 , PMC 304849 (free full text).
- ↑ Y. Oba, N. Yoshida, S. Kanie, M. Ojika, S. Inouye: Biosynthesis of firefly luciferin in adult lantern: decarboxylation of L-cysteine is a key step for benzothiazole ring formation in firefly luciferin synthesis. In: PloS one . Volume 8, number 12, 2013, p. E84023, doi : 10.1371 / journal.pone.0084023 , PMID 24391868 , PMC 3877152 (free full text).
- ^ S. Kanie, T. Nishikawa, M. Ojika, Y. Oba: One-pot non-enzymatic formation of firefly luciferin in a neutral buffer from p-benzoquinone and cysteine. In: Scientific reports . Volume 6, April 2016, p. 24794, doi : 10.1038 / srep24794 , PMID 27098929 , PMC 4838837 (free full text).
- ↑ L. Pinto da Silva, JC Esteves da Silva: Firefly luciferin as a multifunctional chemiluminescence molecule. In: Photochemical & photobiological sciences: Official journal of the European Photochemistry Association and the European Society for Photobiology. Volume 12, Number 9, September 2013, pp. 1615-1621, doi : 10.1039 / c3pp50086a , PMID 23712132 .
- ↑ J. Vieira, L. Pinto da Silva, JC Esteves da Silva: Advances in the knowledge of light emission by firefly luciferin and oxyluciferin. In: Journal of photochemistry and photobiology. B, Biology. Volume 117, December 2012, pp. 33-39, doi : 10.1016 / j.jphotobiol.2012.08.017 , PMID 23026386 .

![\ mathrm {LH_2 + O_2 + ATP \ xrightarrow [Mg ^ {2 +}] {Luciferase} \ oxy \ text {-} L + CO_2 + AMP + PP_i + h \ nu}](https://wikimedia.org/api/rest_v1/media/math/render/svg/910bfa2b41cb3e3e1dbfbafa4289895b5132fa03)