Dammaran
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
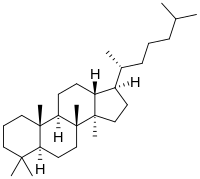
|
|||||||||||||
| General | |||||||||||||
| Surname | Dammaran | ||||||||||||
| other names |
(5 S , 8 R , 9 R , 10 S , 13 R , 14 R , 17 R ) -4,4,8,10,14-pentamethyl-17 - [(2 R ) -6-methylheptan-2-yl ] -2,3,5,6,7,9,11,12,13,15,16,17-dodecahydro-1H-cyclopenta [a] phenanthrene ( IUPAC ) |
||||||||||||
| Molecular formula | C 30 H 54 | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 414.75 g mol −1 | ||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Dammaran is a triterpenoid that is part of some sapogenins , such as the ginsenosides panaxatriol and protopanaxadiol . Dammaran is the main secondary metabolite after ingestion of ginseng ( Panax ginseng ). So far, triterpenoids of the Dammaran type have been described in 136 plant species.
literature
- S. Zhang, Y. Zhao: Drug Metabolism and Pharmacokinetics of Dammarane Triterpenoids. In: Current drug metabolism. Volume 17, Number 9, 2016, pp. 836-848, PMID 27697027 .
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ J. Cao, X. Zhang, F. Qu, Z. Guo, Y. Zhao: Dammarane triterpenoids for pharmaceutical use: a patent review (2005-2014). In: Expert opinion on therapeutic patents. Volume 25, Number 7, July 2015, pp. 805-817, doi : 10.1517 / 13543776.2015.1038239 , PMID 25892194 .
- ↑ J. Ruan, C. Zheng, L. Qu, Y. Liu, L. Han, H. Yu, Y. Zhang, T. Wang: Plant Resources, (13) C-NMR Spectral Characteristic and Pharmacological Activities of Dammarane- Type triterpenoids. In: Molecules. Volume 21, Number 8, August 2016, p., Doi : 10.3390 / molecules21081047 , PMID 27529202 .