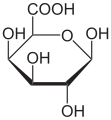
Galacturonic acid
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| Fischer projection , open-chain representation | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Galacturonic acid | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 6 H 10 O 7 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 194.14 g mol −1 | ||||||||||||||||||
| Physical state |
solid (monohydrate) |
||||||||||||||||||
| Melting point |
166 ° C (monohydrate) |
||||||||||||||||||
| solubility |
soluble in water and ethanol , insoluble in diethyl ether |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Galacturonic acid , or GalA for short , is an organic-chemical compound from the group of uronic acids . It occurs naturally in the form of D- galacturonic acid, especially as the main component of pectins (which was first suspected by K. Smolenski in 1924) and is one of the dietary fibers . Formally, D- galacturonic acid can be regarded as an oxidized form of D- galactose . Galacturonic acid salts are known as galacturonates.
α- D- galacturonic acid in Haworth notation
See also
- Pectins (polyuronic acids)
Web links
- Ion chromatographic determination of galacturonic acid in wine: Joachim Weiss: Ion chromatography . John Wiley & Sons, 2012, ISBN 3-527-66080-1 ( limited preview in Google Book Search).
Individual evidence
- ↑ a b Data sheet D - (+) - Galacturonic acid monohydrate, ≥97.0% from Sigma-Aldrich , accessed on May 13, 2017 ( PDF ).
- ↑ a b David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-266.
- ↑ Herbstreith-fox.de: Pectin - the natural product (PDF; 621 kB).