Gentamicins
The gentamicins are a group of structurally very closely related aminoglycoside compounds . Under the generic name gentamicin , a type mixture produced by fermentation is used as a medicinal substance against bacterial infections.
history
The first gentamicins were discovered in 1963 by employees of Schering in New Jersey in the products of the bacterial strain Micromonospora purpurea . The spelling with an " i " is derived from the bacterial origin (M i cromonospora) . Aminoglycoside antibiotics derived from Streptom y ces species, on the other hand, are written with “ y ” (for example Tobram y cin or Streptom y cin ).
Structures and properties
The gentamicins are made up of three hexosamines . These are (see figures, from left to right) gentosamine / garosamine , 2-deoxystreptamine and purpurosamine .
| Gentamicins | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Name (s) | structure |
CAS - number |
PubChem | Molecular formula | Molar mass |
||||
|
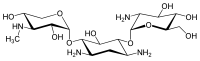
|
13291-74-2 | 86474 | C 18 H 36 N 4 O 10 | 468.50 g mol −1 | ||||
|
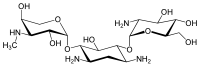
|
||||||||
|
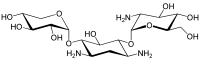
|
55715-66-7 | 86489 | C 17 H 33 N 3 O 11 | 455.46 g mol −1 | ||||
|

|
55715-67-8 | 86490 | C 18 H 36 N 4 O 10 | 468.50 g mol −1 | ||||
|
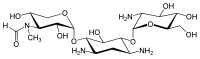
|
||||||||
|
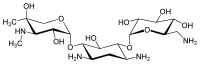
|
36889-15-3 | 37569 | C 19 H 38 N 4 O 10 | 482.53 g mol −1 | ||||
|

|
36889-16-4 | 3034288 | C 20 H 40 N 4 O 10 | 496.55 g mol −1 | ||||
|

|
25876-10-2 | 441305 | C 21 H 43 N 5 O 7 | 477.59 g mol −1 | ||||
|
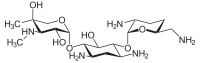
|
26098-04-4 | 72396 | C 19 H 39 N 5 O 7 | 449.54 g mol −1 | ||||
|

|
25876-11-3 | 72397 | C 20 H 41 N 5 O 7 | 463.57 g mol −1 | ||||
|

|
59751-72-3 | C 20 H 41 N 5 O 7 | 463.57 g mol −1 | |||||
|
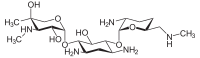
|
52093-21-7 | 107677 | C 20 H 41 N 5 O 7 | 463.57 g mol −1 | ||||
The kanamycins and tobramycin also have similar structures . In sisomicin is 4.5 Dehydrogentamicin-C 1a .
Individual evidence
- ^ Gentamicin . In: Br Med J . 1, No. 5533, January 1967, pp. 158-9. PMID 6015651 . PMC 1840594 (free full text).
- ↑ Ingo Stock: Bacteria Viruses Active Ingredients . Govi-Verlag, 2009, ISBN 9783774111042 .
- ↑ Benveniste R, Davies J: Structure-activity relationships among the aminoglycoside antibiotics: role of hydroxyl and amino groups . In: Antimicrob. Agents Chemother. . 4, No. 4, October 1973, pp. 402-9. PMID 4598613 . PMC 444567 (free full text).
- ↑ Vastola AP Altschaefl J, Harford S: 5-epi-sisomicin and 5-epi-gentamicin B: substrate for aminoglycoside-modifying enzymes did retain activity against aminoglycoside-resistant bacteria . In: Antimicrob. Agents Chemother. . 17, No. 5, May 1980, pp. 798-802. PMID 6967296 . PMC 283878 (free full text).