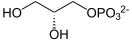
L- glycerol-3-phosphate
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||
| General | |||||||||||||
| Surname | L- glycerol-3-phosphate | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 3 H 9 O 6 P | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 172.07 g mol −1 | ||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
L- glycerol-3-phosphate is a chemical compound from the group of phosphorylated glycerols .
properties
As a chiral molecule, glycerol-3-phosphate has a stereocenter and therefore a D and an L form.
In living beings , the L form occurs for fat formation ( lipogenesis ) . It is formed, among other things, by glycerol-3-phosphate dehydrogenase through the reduction of dihydroxyacetone phosphate (DHAP), which comes from glycolysis . Glycerol-3-phosphate can also be formed from amino acids and from the citrate cycle in the course of gluconeogenesis .
Furthermore, it can be formed with the help of glycerol kinase through the phosphorylation of glycerol , which is produced when fats are broken down ( lipolysis ).
Glycerol-3-phosphate is the starting material in the biosynthesis of glycerolipids . It is acylated in eukaryotes at the sn -1 position by glycerol-3-phosphate-O-acyltransferase in the mitochondrion or in the endoplasmic reticulum , whereupon further acylation takes place at the sn -2 position to form phosphatidic acid .
-
 + Acyl-CoA → lysophosphatidic acid + CoA
+ Acyl-CoA → lysophosphatidic acid + CoA
The glycerol-1-phosphatase hydrolyzes the phosphate group, whereby glycerol is formed.
-
 + H 2 O →
+ H 2 O →  + Pi
+ Pi
The glycerol-3-phosphate shuttle transports glycerol-3-phosphate through the mitochondrial membranes.
Commercial form
The lithium salt of the compound is commercially available.
literature
- IUPAC : Nomenclature of Phosphorus-Containing Compounds of Biochemical Importance (Recommendations 1976).
Web links
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ External identifiers or database links for glycerol-3-phosphate : CAS number: 57-03-4, EC number: 200-307-1, ECHA InfoCard: 100.000.279 , PubChem : 754 , ChemSpider : 734 , Wikidata : Q2739882 .
- ↑ Y. Mugabo, S. Zhao, A. Seifried, S. Gezzar, A. Al-Mass, D. Zhang, J. Lamontagne, C. Attane, P. Poursharifi, J. Iglesias, E. Joly, ML Peyot, A. Gohla, SR Madiraju, M. Prentki: Identification of a mammalian glycerol-3-phosphate phosphatase: Role in metabolism and signaling in pancreatic β-cells and hepatocytes. In: Proceedings of the National Academy of Sciences . Volume 113, number 4, January 2016, pp. E430 – E439, doi : 10.1073 / pnas.1514375113 , PMID 26755581 , PMC 4743820 (free full text).
- ↑ Data sheet sn-Glycerol 3-phosphate lithium salt, ≥95.0% (TLC) from Sigma-Aldrich , accessed on March 24, 2018 ( PDF ).