Glyoxal
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Glyoxal | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 2 H 2 O 2 | |||||||||||||||
| Brief description |
yellow liquid with a characteristic odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 58.04 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.14 g cm −3 |
|||||||||||||||
| Melting point |
|
|||||||||||||||
| boiling point |
|
|||||||||||||||
| Vapor pressure |
|
|||||||||||||||
| solubility |
good in water (600 g l −1 at 20 ° C, glyoxal hydrate) |
|||||||||||||||
| Refractive index |
1.3826 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Glyoxal (according to IUPAC nomenclature : oxalaldehyde or ethanedial ) is an organic-chemical compound from the group of aldehydes . Due to its bifunctionality, it serves as a versatile chemical intermediate with numerous applications. As bulk glyoxal comes as a 40% aqueous solution on the market.
Occurrence
Glyoxal occurs as a trace gas in the atmosphere, as a breakdown product of hydrocarbons. The tropospheric concentrations are usually 0–200 pptv , in polluted regions up to 1 ppbv .
Extraction and presentation
The large-scale production of glyoxal is now practically carried out exclusively by two different processes that have prevailed in terms of sales , selectivity , yield and profitability .
Laporte method
In the Laporte process, ethylene glycol is converted into glyoxal and water by gas phase oxidation with oxygen in the presence of metallic silver or copper catalysts at temperatures of 400–600 ° C. The yield in this process is approx. 70-80%. The complete reaction is carried out in tubular reactors. The work-up takes place with the help of a multi-stage distillation in rectification columns in order to separate off by- products such as formaldehyde .
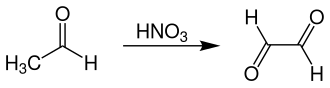
Nitric acid oxidation
The second, industrially important process for the production of glyoxal starts from acetaldehyde , which is oxidized to glyoxal by nitric acid at 40 ° C. Here are yields reached about 70%. The processing takes place with the help of ion exchange resins in order to separate acetic acid , formic acid and glyoxylic acid .
Furthermore, other methods for the synthesis of known, such as the oxidation of acetaldehyde with selenious acid , selenium dioxide or oxygen with palladium - catalysts .
The chemical industry trades and sells bulk goods from Glyoxal exclusively as a 40% aqueous solution . The world's largest manufacturer is BASF SE with its glyoxal plant at the Verbund site in Ludwigshafen in Germany . This has an annual capacity of approx. 80,000 tons of glyoxal, which is offered in tank containers as well as barrel and IBC goods .
properties
Physical Properties
As a pure substance, glyoxal has a density of 1.14 g · cm −3 at 20 ° C, a relative gas density of 2.00 (density ratio to dry air at the same temperature and pressure ) and a vapor pressure of 293 hPa at 20 ° C. A 40% aqueous solution at 20 ° C has a density of 1.27 g · cm −3 and a vapor pressure of 24 hPa. Liquid and solid glyoxal is yellow; the fumes are green.
Chemical properties
Glyoxal is a liquid from the group of (divalent) aldehydes . At 20 ° C in anhydrous form it is liquid, below the melting point it forms yellow crystals. Because of its bifunctionality, it tends to undergo polymerization reactions . For this reason, glyoxal is only sold as a 40% aqueous solution in which it is present as a mixture of dihydrate and various oligomers . In addition, ethandial enters into the reactions typical of aldehydes and can thus be converted into glyoxylic acid by oxidizing agents and then converted into oxalic acid . With reducing agents , it breaks down again into its starting compounds, ethylene glycol or acetaldehyde . Glyoxal enters into an intramolecular Cannizzaro reaction with alkaline compounds and forms glycolates , i.e. salts of glycolic acid . Glyoxal also reacts with ammonia , amines and amides and other nitrogen compounds . In a reaction with urea arise imidazole - derivatives . At 20 ° C, a 40% aqueous solution has a pH of 2.1-2.7.
use
Due to its bifunctionality, glyoxal is widely used as an intermediate in the chemical and processing industries . It is often used for condensation and crosslinking reactions with starch , cellulose , keratin , casein , animal glue and mineral building materials. It also serves as an intermediate in the synthesis of heterocyclic compounds and many other chemicals . In polymers glyoxal improves the dissolving and emulsifying (for. Example, when cellulose ethers and methyl cellulose ). It is also used in cosmetics and personal care products. Glyoxal is also used in textile , paper and leather finishing and in the mineral oil industry as an H 2 S scavenger , water conditioner, biocide or oil field chemical. In the health care and veterinary hygiene glyoxal used for formulation of disinfectants .
safety instructions
Glyoxal is mainly absorbed through the respiratory tract and skin . This can cause irritation to the mucous membranes and skin. In addition, a skin-sensitizing effect, which can lead to chronic irritation and permanent skin damage, was found. Renal dysfunction and damage to the pancreas have been demonstrated after prolonged exposure or direct ingestion of high concentrations . A mutagenic effect was shown for the genotoxicity of prokaryotes and eukaryotes , a carcinogenicity is therefore strictly assumed, but has not yet been officially confirmed. With an ignition temperature of 285 ° C of the 40% aqueous solution, the substance falls into temperature class T3.
Individual evidence
- ↑ a b c d e f Entry on glyoxal. In: Römpp Online . Georg Thieme Verlag, accessed on February 11, 2019.
- ↑ a b c d e f g h i j k l m n o Entry on glyoxal, aqueous solution in the GESTIS substance database of the IFA , accessed on January 8, 2019(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-270.
- ↑ Entry on glyoxal in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on January 8, 2019. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ M. Vrekoussis, F. Wittrock, A. Richter, JP Burrows: Temporal and spatial variability of glyoxal as observed from space. In: Atmos. Chem. Phys. 9, 2009, pp. 4485-4504, doi: 10.5194 / acp-9-4485-2009 .
- ^ Rainer Volkamer et al .: A missing sink for gas ‐ phase glyoxal in Mexico City: Formation of secondary organic aerosol. In: Geophysical Research Letters . 34, 19, 2007, doi: 10.1029 / 2007GL030752 .
- ↑ a b Georges Mattioda, Alain Blanc: glyoxal. In: Ullmann's Encyclopedia of Industrial Chemistry . Wiley ‐ VCH Verlag GmbH & Co. KGaA., October 15, 2011, p. 3, doi : 10.1002 / 14356007.a12_491.pub2 (section “4. Production”).
- ↑ a b glyoxal 40%. In: BASF product search. BASF SE, 2014, accessed January 8, 2019 .
- ↑ Beyer-Walter, Textbook of Organic Chemistry, 23rd Edition, S. Hirzel Verlag 1998 ISBN 3-7776-0808-4