Immunoglobulin G
Immunoglobulin G ( IgG ) - the main component of the gamma globulin fraction of serum electrophoresis - are the antibodies ( immunoglobulins ) of class G, which are primarily effective against viruses and bacteria . The formation of immunoglobulins, also known as gamma globulins , is part of the humoral immune defense , i.e. the non-cell-bound immune defense through substances soluble in the blood. From a biochemical point of view, these are special proteins, the glycoproteins . They are produced by B lymphocytes or plasma cells after contact with an antigen .
Newborns cannot produce their own IgG at first and are temporarily dependent on the mother's antibodies. In some cases, IgG can already be detected in the fetal blood. It passes through the placenta with the help of certain transport receptors.
function
Type G immunoglobulins are used for neutralization , opsonization , complement activation and antibody-dependent cell-mediated cytotoxicity . The immunoglobulins themselves cannot destroy the target in question; rather, they have the task of marking the targets and making them more vulnerable to other defense systems. However, since an IgG can only bind to a single antigen, antibody formation belongs to the specific part of the immune system. Certain plasma cells produce very specific antibodies that are directed against a specific antigen. One therefore speaks of the function of the plasma cell as a memory cell . Since IgG are only formed after a class change , they are counted as part of the secondary humoral immune response due to the delayed occurrence upon initial contact with an antigen. In the event of renewed contact, they will be detectable after 24 to 48 hours. In the context of a vaccination , the formation of antibodies against a certain antigen can be "learned" by the plasma cells and can enable the organism to quickly and in large numbers to form specifically acting antibodies when it comes into contact with the same antigen again after many years.
In the spleen , the immunoglobulins are partially broken down, releasing the tissue hormone tuftsin (a tetrapeptide).
Subtypes
There are four subtypes of IgG, IgG1-4. IgG is the most common type of immunoglobulins in the human bloodstream .
| Surname | Proportion of IgG | Placental penetration | Complement activation | Binding to Fc receptors on phagocytes | Plasma half-life |
| IgG1 | 66% | Yes (1.47) * | second highest | high affinity | 21 days |
| IgG2 | 23% | No (0.8) * | third highest | very low affinity | 21 days |
| IgG3 | 7% | Yes (1.17) * | highest | high affinity | 7 days |
| IgG4 | 4% | Yes (1.15) * | no | medium affinity | 21 days |
| * Ratio of the concentrations of umbilical cord to maternal blood. | |||||
structure
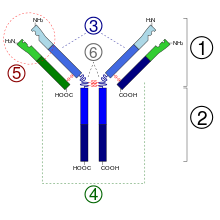
The antibody consists of two long (“heavy”) and two short (“light”) protein chains and is shaped like a “Y”. The heavy chains have a molecular mass of 50,000 Daltons and are always assigned to the class gamma for "immunoglobulin G", the light chains have a molecular mass of 25,000 Daltons. The total molar mass is around 150,000 Daltons (2 × 50,000 + 2 × 25,000). At the short ends of the Y are the binding sites that can bind to antigens (foreign bodies, for example specific surface structures of bacterial cells) or haptens . One heavy and one light chain are linked by a disulfide bridge. Both heavy chains are linked by two disulfide bridges. The antigen-binding portion of IgG ( Fab fragment ) is very variable. It has been calculated that about 10 6 to 10 9 chemically different Fab segments are possible. The corresponding gene segments are subject to the corresponding necessary mutations during life. The binding to surface antigens triggers further immunological reactions which can lead to the destruction of the affected cells or antigen-presenting structures. After glycosylation of the IgG at position N297 of the heavy chain, its affinity for its receptor Fcγ receptor and for the complement receptor increases . The IgG are sometimes changed differently by different post-translational modifications .
The heavy chains of immunoglobulins of class G (gamma) are divided into four isotypic subclasses (gamma 1-4). The light chains come in two types (kappa and lambda).
Certain enzymes (e.g. papain or pepsin ) can split the IgG antibodies into two types of fragments. The three fragments obtained after cutting with papain are:
- Fab: Two fragments which contain a monovalent antigen binding site ( fragment, antigen binding ).
- Fc: A fragment that can bind to complement proteins or cellular Fc receptors ( fragment, crystallizable ).
If you cut the immunoglobulin with pepsin, then you get:
- F [ab] 2: A fragment which contains a divalent antigen binding site and is held together by the disulfide bridges under the hinge region
- Fc: There is no Fc fragment, since the heterodimer is no longer held together by the disulfide bridges under the hinge region. The result is two polypeptide chains
If you cut the immunoglobulin with plasmin, you get:
- Facb: A fragment which contains a divalent antigen binding site (paratope) and is still held together by the polysaccharide component and disulfide bridges in the Fc part
- pFc: Two polypeptide chains that are not held together by bonds.
pathology
The body's own antigens are regarded as “own” within the framework of self-tolerance and normally do not lead to specific antibody formation against these antigens. In the case of autoimmune diseases , however, antibodies can be formed against the body's own structures that have a disease value.
Disorders of antibody IgG formation are known as gammopathies . The Hypogammaglobulinaemia refers to a pathologically insufficient production of IgG antibodies. The agammaglobulinemia means the failure of antibody formation. Severe protein deficiency ( inanition , long periods of hunger) or severe kidney diseases (nephrotic syndrome) can impair the formation of antibodies. A reduction in IgG formation leads to sometimes serious infectious diseases. IgG are involved in some inflammatory bowel diseases and allergies of type II and III.
IgG4 autoimmune diseases have been described as a separate disease group.
laboratory
The immunoglobulins G can be identified in conventional immunoelectrophoresis or, better, in immunofixation electrophoresis . Human blood contains around 8 grams of IgG per liter and represents 11–18% of the total protein in the blood.
Applications
Type G immunoglobulins are used medically, among other things, for passive immunization and immunotherapy . In biochemistry they are used for cell depletion and for immunostaining . Some doctors offer immunoglobulin G (IgG) testing to clarify symptoms that could be due to a food allergy. The IGeL monitor of the MDS (Medical Service of the Central Association of Health Insurance Funds) rates the immunoglobulin G determination for diagnosing a food allergy as "negative". The IgG test is not at all suitable for detecting food allergies. Another type of immunoglobulins is responsible for allergies. In addition, the studies examined did not show any indications of a benefit, since a high concentration of IgG is not associated with allergy symptoms. However, there are indications of considerable damage, as the test can lead to an unnecessary restriction in diet, in extreme cases even to malnutrition.
Web links
- Article accompanying a radio report (WDR 5, “Leonardo”) that dealt critically with IgG tests. ( Memento from September 30, 2007 in the Internet Archive )
- Deutsches Ärzteblatt: Food allergies and intolerance: Proven instead of non-evaluated diagnostics (July 8, 2005)
Individual evidence
- ↑ S. Bournazos, DJ Dilillo, JV Ravetch: The role of Fc-FcγR interactions in IgG-mediated microbial neutralization. In: The Journal of experimental medicine. Volume 212, number 9, August 2015, pp. 1361-1369, doi : 10.1084 / jem.20151267 , PMID 26282878 , PMC 4548051 (free full text).
- ^ V. Trivedi, SC Zhang, AB Castoreno, W. Stockinger, EC Shieh, JM Vyas, EM Frickel, A. Nohturfft: Immunoglobulin G signaling activates lysosome / phagosome docking. In: Proceedings of the National Academy of Sciences . Volume 103, number 48, November 2006, pp. 18226-18231, doi : 10.1073 / pnas.0609182103 , PMID 17110435 , PMC 1838734 (free full text).
- ^ A b I. Quast, JD Lünemann: Fc glycan-modulated immunoglobulin G effector functions. In: Journal of clinical immunology. Volume 34 Suppl 1, July 2014, pp. S51-S55, doi : 10.1007 / s10875-014-0018-3 , PMID 24760108 .
- ↑ M. Biburger, A. Lux, F. Nimmerjahn: How immunoglobulin G antibodies kill target cells: revisiting an old paradigm. In: Advances in immunology. Volume 124, 2014, pp. 67-94, doi : 10.1016 / B978-0-12-800147-9.00003-0 , PMID 25175773 .
- ^ FA Bonilla: Pharmacokinetics of immunoglobulin administered via intravenous or subcutaneous routes. In: Immunology and allergy clinics of North America. Volume 28, Number 4, November 2008, pp. 803-19, ix, doi : 10.1016 / j.iac.2008.06.006 , PMID 18940575 .
- ↑ Hashira S, Okitsu-Negishi S, Yoshino K: Placental transfer of IgG subclasses in a Japanese population . In: Pediatr Int . 42, No. 4, August 2000, pp. 337-42. doi : 10.1046 / j.1442-200x.2000.01245.x . PMID 10986861 .
- ^ J. Lu, PD Sun: Structural mechanism of high affinity FcγRI recognition of immunoglobulin G. In: Immunological reviews. Volume 268, Number 1, November 2015, pp. 192-200, doi : 10.1111 / imr.12346 , PMID 26497521 .
- ↑ QM Hanson, AW Barb: A perspective on the structure and receptor binding properties of immunoglobulin G Fc. In: Biochemistry. Volume 54, number 19, May 2015, pp. 2931-2942, doi : 10.1021 / acs.biochem.5b00299 , PMID 25926001 , PMC 4894528 (free full text).
- ↑ LK Hmiel, KA Brorson, MT Boyne: Post-translational structural modifications of immunoglobulin G and their effect on biological activity. In: Analytical and bioanalytical chemistry. Volume 407, Number 1, January 2015, pp. 79-94, doi : 10.1007 / s00216-014-8108-x , PMID 25200070 .
- ↑ C. Cai, J. Shen, D. Zhao, Y. Qiao, A. Xu, S. Jin, Z. Ran, Q. Zheng: Serological investigation of food specific immunoglobulin G antibodies in patients with inflammatory bowel diseases. In: PloS one. Volume 9, number 11, 2014, p. E112154, doi : 10.1371 / journal.pone.0112154 , PMID 25393003 , PMC 4230978 (free full text).
- ↑ IGeL-Monitor, evaluation of the immunoglobulin G determination for the diagnosis of a food allergy . Retrieved October 15, 2018.
