Coordinative bond
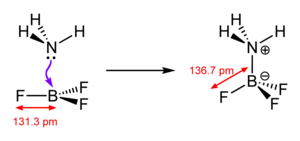
A coordinative bond (also donor-acceptor bond or obsolete dative bond ) is a special type of bond in complex chemistry . Such a bond exists when the binding electrons in an electron pair bond come from only one of the two binding partners. The molecule or ion with a lack of electrons is called the acceptor (= Lewis acid ), and the one with the free electrons is called the donor (= Lewis base ). In old textbooks, this bond is sometimes still indicated by an arrow in the direction of the acceptor. These representations are out of date. A coordinative bond, like any other covalent bond, is drawn as a line (see e.g. adjacent sketch).
A typical example is ammonia (NH 3 ), which makes its lone pair of electrons available for a coordinative bond, see H 3 N-BF 3 in the adjacent sketch. Formally, the nitrogen gives an electron to the boron, whereby the former (generally: the donor) receives a formal positive, the latter (generally: the acceptor) receives a formal negative charge. Note that these formal charges have nothing to do with the actual charge distribution: Since nitrogen has a much higher electronegativity than boron (3.07 compared to 2.01), the electrons are more likely to stay with nitrogen.
Individual evidence
- ↑ Entry on coordination . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.C01329 Version: 2.3.3.
- ↑ Erwin Riedel: Allgemeine und Anorganische Chemie , Walter de Gruyter Verlag (2018), Section 2.2.3, ISBN 3-11-016415-9 .