Methemoglobin
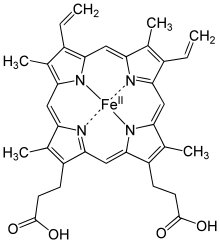
Methemoglobin (Met-Hb, also hemiglobin or ferrihemoglobin ) is a derivative of hemoglobin , the blood pigment in the red blood cells of vertebrates. It is formed when the divalent iron in hemoglobin (Hb) is oxidized to trivalent iron (Fe 2+ → Fe 3+ ). This happens in the erythrocytes under physiological conditions by the accumulation of oxygen in the sense of auto-oxidation .
Methemoglobin now has the ligand water instead of oxygen. However, it ensures that hemoglobin in its environment can still absorb oxygen but can no longer release it. Methemoglobin is formed, among other things, in the event of poisoning by oxidizing agents such as nitrites and hydrogen peroxide or by aromatic amino and nitro compounds such as aniline and nitrobenzene . A number of drugs are also potential methaemoglobin builders: dapsone, prilocaine , sulfonamides , nitroglycerin , nitroprusside and nitric oxide (NO).
The enzyme methemoglobin reductase reduces methemoglobin back to hemoglobin, so the methemoglobin content in human blood generally does not exceed 1.5%. There is a particular sensitivity to methaemoglobin formers in infants , since the activity of methaemoglobin reductase is not fully developed until the middle of the first year of life.
From a methemoglobin content of 15% cyanosis can be observed, from 30 to 40% there are signs of oxygen deficiency in the tissue, especially in the brain (confusion, dizziness, impaired consciousness). Values between 60 and 80% are fatal. It should be noted that the oxygen saturation measured by pulse oximetry does not fall below 80 to 85% even with very high methaemoglobin concentrations .
Therapy is carried out by intravenous administration of methylene blue . This substance is itself a methaemoglobin generator, but can accelerate the enzymatic regression of high methaemoglobin concentrations, whereby an equilibrium is established at around 10% methaemoglobin content. Another antidote is toluidine blue , also known as tolonium chloride. The exchange transfusion is a therapeutic option in very pronounced cases.
In the case of cyanide poisoning, methaemoglobin- forming agents ( 4-dimethylaminophenol , DMAP for short) are given in addition to sodium thiosulphate and hydroxycobalamin . Cyanide has a high affinity for ferric ions. These ions are present in cytochrome oxidase (enzyme of the cells' respiratory chain , located in the mitochondria ). If the iron (III) ion is complexed by cyanide, this is reversibly inhibited so that metabolic energy ( ATP ) is no longer provided. Through the formation of methemoglobin, the cyanide is held in the blood through complex formation with its iron (III) ions, so that the transfer into the tissue and thus the inhibition of cytochrome oxidase is prevented. The actual detoxification takes place via the rhodanase to thiocyanate, which is accelerated by the therapeutic administration of sulfur compounds (sodium thiosulfate).
Bedside test
In the bedside test for methaemoglobinemia, one drop each of normal and abnormal blood is placed on a filter paper and compared after one minute. Methemoglobin blood remains brown in color.
literature
- Georg Löffler , Petro E. Petrides (Ed.): Biochemistry and Pathobiochemistry. 7th edition. Springer Medizin Verlag, Heidelberg 2003, ISBN 3-540-42295-1 .
- Rolf Rossaint, Christian Werner, Bernhard Zwißler (ed.): The anesthesiology. Springer-Verlag, Berlin 2004, ISBN 3-540-00077-1 .
Individual evidence
- ↑ Joachim Rassow et al: Biochemistry. 1st edition. Thieme Verlag Stuttgart 2006, ISBN 3-13-125351-7 .
- ↑ Saralyn R. Williams: methemoglobin. In: Luis Ling et al.: Toxicology Secrets. Hanley & Belfus, Philadelphia 2001, ISBN 1-56053-410-9 , p. 181.
- ^ KA Evelyn, HT Malloy: Micro determination of methaemoglobin and sulphaemoglobin in a sample of blood. In: British Journal of Hematology . vol. 126 (1938), pp. 555-652.