Sodium naphthalide
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Sodium naphthalide | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 10 H 8 Na | ||||||||||||||||||
| Brief description |
dark green solution |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 151.16 | ||||||||||||||||||
| Physical state |
liquid (solution in ethers) |
||||||||||||||||||
| solubility |
soluble in ethers except in diethyl ether |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
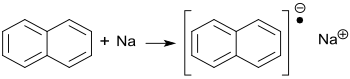
Sodium naphthalide is a 1: 1 ion-pair from the sodium - cation and naphthalene - radical anion , which is characterized by its dark green color in substance and in solution. Because of the instability of the solid substance and its instability to air and moisture, sodium naphthalene must be dissolved in a suitable solvent such as e.g. B. Tetrahydrofuran THF or ethylene glycol dimethyl ether glyme can be freshly prepared. As a radical anion, sodium naphthalene is used as a base and reducing agent and - similar to organolithium compounds such. B. Butyllithium - as an initiator for anionic polymerization .
Occurrence and representation
As early as 1914, Wilhelm Schlenk and co-workers found that the tricyclic aromatic anthracene in diethyl ether reacts with metallic sodium to form the blue sodium anthracenide , but not the bicyclic naphthalene to form the corresponding sodium naphthalide. If, however, bare pieces of sodium are added to a solution of naphthalene in dry ethylene glycol dimethyl ether under a dry nitrogen atmosphere at temperatures between −10 and +30 ° C and stirred for 2 h, a dark green solution of naphthalene sodium is formed.
Sodium naphthalide is also produced in quantitative yield in THF, with ethers such as THF and glyme solvating the ion pair formed .
properties
In THF , glyme and dioxane, sodium naphthalide forms dark green (absorption maxima λ max at 463 and 735 nm) and electrically conductive solutions, from which a deep green solid is formed when the solvent is removed, which spontaneously decomposes into naphthalene and metallic sodium. The additional electron in the radical anion C 10 H 8 - is taken up in the lowest unoccupied π molecular orbital and is delocalized over all ring atoms of the naphthalene .
The radical character of sodium naphthalide with a Landé factor g = 2 for pure spin angular momentum is shown in a strong electron spin resonance (ESR) signal with a redox potential of approx. −2.5 volts compared to a standard hydrogen electrode NHE.
Sodium naphthalide is rapidly discolored in air.
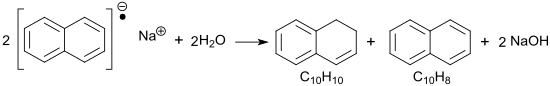
Sodium naphthalide reacts with water as a strong base with the formation of hydrogen to sodium hydroxide NaOH and the hydrogenation product dihydronaphthalene C 10 H 10 .
With other OH-acids , such as. B. alcohols , or CH-acidic compounds such. B. acetylene , are formed analogously to dihydronaphthalene and sodium alcoholates or sodium acetylide.
Therefore, the manufacture and handling of sodium naphthalide must take place with the strict exclusion of oxygen and water.
Since sodium naphthalene dissolved in ethylene glycol dimethyl ether gradually attacks the solvent with the formation of dihydronaphthalene and methyl vinyl ether , the compound should always be freshly prepared (preferably in THF).
Applications
The pronounced basicity of sodium naphthalide can be used to abstract protons in the α-position of CH-acidic nitriles , which can be alkylated with alkyl halides in high yield .
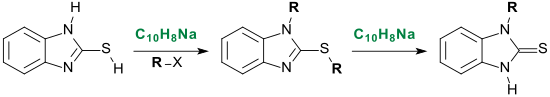
Similarly, sodium naphthalide abstracts protons from 2-mercaptobenzimidazole in the thiol form and the anion reacts quantitatively with alkyl halides, e.g. B. Benzyl chloride ( R = benzyl) to 1-benzyl-2-benzylthiobenzimidazole.
Addition of further sodium naphthalide produces 1-benzylbenzimidazoline-2-thione in 93% yield.
The high reactivity of sodium naphthalide towards organic halogen compounds is demonstrated by the practically complete dechlorination (99.5%) of polychlorinated biphenyls at 22 ° C within 15-30 minutes.
Perfluorinated surfaces, such as B. of polytetrafluoroethylene PTFE (Teflon®), naphthalene sodium in glymes [H 3 C (OCH 2 CH 2 ) n OCH 3 with n = 1, 2, or 4] quickly turns brown to black and forms double bonds and oxygen functions such as C = O and OH groups attacked or etched (engl. etching ). This makes the surface wettable and can be connected or glued to other materials.
An interesting application of sodium naphthalide is the stereoselective conversion of geraniol into linalool . First, the geraniol is oxidized to 2,3-epoxygeraniol by means of a Sharpless epoxidation with vanadyl acetylacetonate / tert-butyl hydroperoxide , then the hydroxyl group is protected by a mesyl group and then the reductive cleavage of the epoxide with elimination of the mesyl group to linalool.
The overall yield over all three stages is 73%.
Like the mesyl group, other protecting groups , such as. B. the benzyl group or tosyl group , under mild conditions quantitatively using sodium naphthalide, e.g. B. from protected (-) - menthol can be removed.
Sodium naphthalide in THF is suitable as an initiator for the anionic living polymerization of vinyl monomers such as styrene to polystyrene .
The initially formed red styrene radical anion dimerizes to a dianion, which grows through the addition of further styrene monomers to both chain ends without chain termination or transfer reactions taking place. The polymers obtained are distinguished by a narrow molar mass distribution and - in the case of block copolymers A n B m - defined sequence lengths n and m.
Individual evidence
- ↑ a b c d e f g N.D. Scott, JF Walker, VS Hansley: Sodium Naphthalene. I. A New Method for the Preparation of Addition Compounds of Alkali Metals and Polycyclic Aromatic Hydrocarbons . In: J. Am. Chem. Soc. tape 58 , no. 12 , 1936, pp. 2442–2444 , doi : 10.1021 / ja01303a022 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b Preliminary experiment: preparation of sodium naphthalide (NaC 10 H 8 ). University of Wuppertal, accessed February 8, 2020 .
- ^ GA Molander, CR Harris: Sodium Naphthalenide . In: e-EROS Encyclopedia Of Reagents for Organic Synthesis . 2001, doi : 10.1002 / 047084289X.rs091 .
- ↑ W. Schenk, J. Appenroth, A. Michael, A. Thal: About metal additions to multiple bonds . In: Ber. German chem. Ges. Band 47 , no. 1 , 1914, p. 473-490 , doi : 10.1002 / cber.19140470177 .
- ^ JJ Zuckerman (Ed.): Inorganic Reactions and Methods, Volume 11 . VCH Verlagsgesellschaft mbH, Weinheim 1988, ISBN 0-89573-250-5 , p. 162 .
- ^ NG Connelly, WE Geiger: Chemical redox agents for organometallic chemistry . In: Chem. Rev. Band 96 , no. 2 , 1996, p. 877-910 , doi : 10.1021 / cr940053x .
- ↑ HC Wang, G. Levin, M. Swarc: Production of hydrogen from interaction of an anion radical and water. Comment . In: J. Am. Chem. Soc. tape 100 , no. 12 , 1978, p. 3969-3969 , doi : 10.1021 / ja00480a074 .
- ^ NG Connelly, WE Geiger: Chemical Redox Agents for Organometallic Chemistry . In: Chem. Rev. Band 96 , 1996, pp. 877-910 , doi : 10.1021 / cr940053x .
- ^ Leopold Horner , H. Güsten: Naphthalene sodium as a reactive metalating agent . In: Justus Liebigs Ann. Chem. Band 652 , no. 1 , 1962, pp. 99-107 , doi : 10.1002 / jlac.19626520114 .
- ↑ TR Lee, K. Kim: A facile one pot synthesis of 1-alkylbenzimidazoline-2-thione . In: J. Heterocycl. Chem. Band 26 , no. 3 , 1989, pp. 747-751 , doi : 10.1002 / jhet.5570260344 .
- ↑ JG Smith, GL Bubbar: The chemical destruction of polychlorinated biphenyls by sodium naphthalenide . In: J. Chem. Tech. Biotechnol. tape 30 , no. 1 , 1980, p. 620-625 , doi : 10.1002 / jctb.503300181 .
- ↑ FluoroEtch® Safety Solvent. Acton Technologies, Inc., July 21, 2016, accessed February 12, 2020 .
- ↑ Barry Sharpless , RC Michaelson: High stereo- and regioselectivities in the transition metal catalyzed epoxidations ob olefinic alcohols by tert-butyl hydroperoxide . In: J. Am. Chem. Soc. tape 95 , no. 18 , 1973, p. 6136-6137 , doi : 10.1021 / ja00799a061 .
- ↑ A. Yasuda, H. Yamamoto, Hitoshi Nozaki : A stereoselective 1,3-transposition of allyllic alcohols . In: Tetrahedron Lett. tape 17 , no. 30 , 1976, p. 2621-2621 , doi : 10.1016 / S0040-4039 (00) 91750-7 .
- ↑ WD Closson, P. Wriede, S. Bank: Reductive cleavage of toluenesulfonates with sodium naphthalene . In: J. Am. Chem. Soc. tape 88 , no. 7 , 1966, pp. 1581–1583 , doi : 10.1021 / ja00959a067 .
- ↑ Michael Szwarc , M. Levy, R. Milkovic: Polymerization initiated by electron transfer to monomer. A new method of formation of block copolymers . In: J. Am. Chem. Soc. tape 78 , no. 11 , 1956, pp. 2656-2657 , doi : 10.1021 / ja01592a101 .
- ↑ K. Matyjaszewski, AEH Müller (ed.): Controlled and living polymerizations: from mechanisms to applications . Wiley-VCH, Weinheim 2009, ISBN 978-3-527-32492-7 .