Nerve conduction velocity
Nerve conduction velocities , abbreviated NLG , indicate how quickly electrical impulses are transmitted along the nerve fibers . For this purpose - as defined in physics - the quotient is formed from the location difference and the time difference.
The conduction speeds of nerve fibers are different; they mainly depend on the respective caliber of the nerve cell process ( axon ) and the special formation of a glial cell envelope ( myelin sheath ). In humans, thin unmyelinated (medullary) nerve fibers conduct the excitation impulses at around 1 m / s ( meters per second ), whereas thick and myelinated (medullary) fibers conduct them much faster at around 100 m / s.
Biological foundations
In the conduction structures of the peripheral and central nervous system , its nerves or pathways , bundles of nerve fibers run side by side . A nerve fiber consists of an elongated extension of a nerve cell and its covering, which is formed by glial cells . The enclosed nerve cell process is also called the axis cylinder or axon . Axons can not only be of different lengths, but also of different thicknesses. The larger the axon diameter, the larger the diameter of the nerve fiber. Thicker axons conduct faster than thin ones.
In vertebrates like humans, a special form of envelope can be formed around axons in a further development step. To do this, glial cells wrap themselves tightly around a nerve cell extension so that several layers of their cell membrane envelop it. Such glial sheaths are called myelin sheaths . Lined up in a row, they additionally isolate the axon in sections and thus allow a special form of excitation conduction . Here, action potentials nurmehr leaps ( saltatory up) to those Axonmembranregionen which are exposed between the insulating myelin sheath sections, and (in their gaps Ranviersche Schnürringe regularly follow each other). Therefore, myelinated nerve fibers conduct faster than myelinated nerve fibers.
The myelin sheaths only develop function-dependently around axons of larger diameter during a maturation process and are stronger in myelinated nerve fibers than in myelinated nerve fibers. Thin nerve fibers, on the other hand, remain marrowless, even in the brain. The conduction speed is doubled by myelination for a 1 μm thin fiber, and approximately eightfold for 10 μm.
Increasing the conduction speed enables rapid movement sequences even when signals in a nerve cell are conducted over relatively long distances, as in larger animals. While cephalopods such as squids develop giant axons that are almost 1 mm thick , which continuously conduct an action potential (AP) at around 60 m / s, vertebrates form myelin sheaths for this purpose, through which an AP is passed saltatorily at a similar speed, although the nerve fiber is only one hundredth is so thick.
Physical basics
From a physical point of view, the axon of a nerve fiber consists of a cell membrane as the insulating covering, the axolemm , and a saline solution as the conductive content, the axoplasm. An applied voltage is therefore - as with any electrical cable - carried on according to electrodynamic laws. With a metallic conductor, impulses can be transmitted over long distances at the speed of electric fields close to the speed of light . However, since the nerve cell membrane is only an incomplete insulator and the electrolyte has a relatively high electrical resistance compared, for example, to a copper wire, there is a considerable voltage drop along the nerve fiber. Therefore, nerve impulses can only be passed electrotonically over a very short distance .
An additional process is therefore necessary to pass action potentials along a longer nerve cell extension - changing the ion permeability via voltage-dependent ion channels in the membrane - with which an action potential can be rebuilt . But this is a relatively slow, active process that requires metabolic energy. In mammalian nerve fibers, it either progresses continuously or jumps in sections. The resulting speeds of an impulse line are between 0.2 and 120 m / s, with high line speeds only being achieved with saltatory excitation lines. Because of the molecular structures involved, there is also a clear temperature dependence. In the physiological range, the nerve conduction speed increases by around 1–2 m / s per degree Celsius.
Axon thickness and nerve conduction velocity
→ Main article: conduction of excitation
Thick axons or axis cylinders transmit with higher nerve conduction velocities than thin ones because of the more favorable ratio between the conductive volume ( ) and the membrane surface area ( ), which increases proportionally to the diameter ( ). However, this geometric relationship only applies to the longitudinal resistance or the membrane longitudinal constant .
Nerve conduction in the peripheral nervous system
Nerve fibers of nerves of the peripheral nervous system can be differentiated according to various criteria. A structural criterion is, for example, the thickness and structure of a nerve fiber. Other criteria are for example the conduction speed or the functional assignment of the respective nerve fiber.
Classification of the line speed according to Erlanger / Gasser
| Fiber type / class (according to Erlanger / Gasser ) | Line speed | diameter | efferent to: | afferent of / (classification according to Lloyd / Hunt): |
|---|---|---|---|---|
| Aα | 60-120 m / s | 10-20 µm | Skeletal muscle ( extrafusal ) | Skeletal muscle: muscle spindle (Ia), Golgi tendon organ (Ib) |
| Aβ | 40-90 m / s | 7-15 µm | Skin receptors (touch, pressure) (II) | |
| Aγ | 20-50 m / s | 4-8 µm | Skeletal muscle ( intrafusal ) | |
| Aδ | 10-30 m / s | 2-5 µm | Skin receptors (temperature, rapid pain) (III) | |
| B. | 5-20 m / s | 1-3 µm | Preganglionic visceroefferents | |
| C (without myelin sheath) | 0.5-2 m / s | 0.5-1.5 µm | Postganglionic viscera efferents | slow pain, thermoreceptors (IV) |
Nerve conduction in the central nervous system
The same principles can be found in the central nervous system - the spinal cord and the brain. Especially the nerve fibers of long tracts are myelinated. However, the medullary sheath in the CNS is formed by oligodendrocytes and not by Schwann cells . There are also differences in the structure of the enveloping glial processes and in the components of the myelin . The measurement of the conduction velocities takes place here on the basis of evoked potentials and magnetic stimulation.
Measurement
The measurement of nerve conduction velocities is a standard neurophysiological examination in neurology . Here, however, it is not the nerve conduction velocity of a single nerve fiber that is measured, but the sum of the responses from all fibers of a nerve. By definition, the fastest recognizable answer is used to determine the speed. In reality, the fibers of a nerve conduct at different speeds, which, if properly analyzed, can provide further diagnostic information.
The measurement takes place by means of electrical impulse introduction / readout, measured along a nerve.
A special case is the measurement of the motor transfer time . Since the measurable changes in tension of a nerve on the skin surface are very small and therefore error-prone, with motor nerves one helps oneself to stimulate the nerve but derive the muscle's response. Since muscles with many muscle fibers deliver a much higher measurable tension (factor 1: 1000), this is easily possible. However, the time between stimulus and muscle response ( latency ) is not only influenced by the nerve conduction time, but also the transmission time to the muscle via the motor end plate (approx. 0.8 ms) and the conduction time on the muscle fiber membrane (a few ms). The total time is called the motor transition time. By stimulating the nerve at two different locations with a constant deflection position above the muscle, a 'real' nerve conduction velocity can then be determined by calculating the difference.
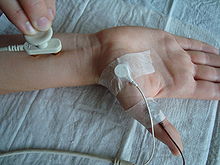
Figure 2: Measurement of the motor transfer time. Stimulation of the median nerve with two poles of a gold contact electrode on the surface of the skin on the wrist, close to the carpal tunnel ; Measurements are made with an electrode glued over the abductor pollicis brevis muscle (ball of the thumb) and a reference electrode on the thumb. For example, a rectangular pulse with a pulse duration of 200 microseconds and a current strength between 3 and 20 mA are set for the stimulus, depending on the location of the stimulation and the thickness and nature of the tissue.
Image 3: The results of two stimuli are shown. The upper trace begins on the left at the time of the stimulus (beginning of the square pulse). From there, the beam travels to the right at the set writing speed of, for example, 5 ms / division. At division 1 (corresponding to a latency of 5 ms) the beginning of the electrical muscle response can be seen as a downward deflection (contrary to convention, negative voltages are shown upward in electrophysiology as standard). This is marked with the left marker. A similar rash can be seen in the lower track, but a little later (right marker). Apparently the stimulus electrode was placed on the nerve further away from the muscle. The time difference between the markers gives the nerve conduction time. The difference in location between the stimulus points is measured with a tape measure, for example. The quotient of the difference in location and time then gives the nerve conduction velocity.
indication
A common indication for measuring nerve conduction velocities is suspicion of polyneuropathy . In this disease, the isolation of the nerves ( myelin ) and / or the nerve process ( axons ) is impaired . As a result of the damage, a reduced nerve conduction velocity can be measured. In carpal tunnel syndrome, the local pressure on the wrist damages the isolation of the median nerve, so that the distal motor latency is significantly increased.
See also
- Electoneurography (ENG)
Individual evidence
- ↑ Excerpt from the classifications according to Erlanger / Gasser 1939 and Lloyd / Hunt 1943 ( Memento of the original from May 7, 2015 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice.
- ^ A b c Josef Dudel, Randolf Menzel, Robert F. Schmidt (eds.): Neuroscience: From Molecule to Cognition. 2nd Edition. Springer-Verlag, 2013, ISBN 978-3-642-56497-0 , p. 113. (online)
- ^ A b Alfred Benninghoff: Macroscopic and microscopic anatomy of humans. Volume 3: Nervous System, Skin and Sensory Organs . Urban & Schwarzenberg, Munich 1985, ISBN 3-541-00264-6 , p. 17f.