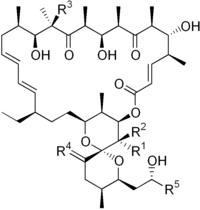
Oligomycin A
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
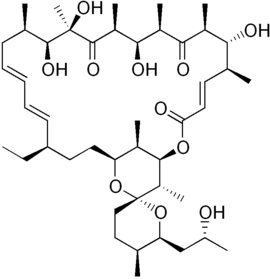
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Oligomycin A | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 45 H 74 O 11 | |||||||||||||||
| Brief description |
White dust |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| Drug class | ||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 791.06 g mol −1 | |||||||||||||||
| Melting point |
140-141 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Oligomycin A is an antibiotic produced by Streptomyces from the group of macrolides , which can be toxic to other organisms. Oligomycin A is the best known representative of the oligomycins.
effect
Oligomycin A is an inhibitor of ATP synthase . In studies of oxidative phosphorylation , it is used to inhibit level 3 respiration, i.e. ADP-induced phosphorylation. Oligomycin A inhibits ATP synthase by blocking the subunit F o of the proton channel (o for oligomycin), which is required for the oxidative phosphorylation of ADP to ATP (energy production).
The inhibition of ATP synthesis by oligomycin A significantly reduces the flow of electrons through the electron transport channel. However, the flow of electrons does not come to a complete standstill due to "proton leaks" or mitochondrial decoupling. These proton leaks come about through facilitated diffusion using an uncoupling protein such as thermogenin (also known as UCP1 ).
Oligomycins
Web links
- Spektrum.de, Lexicon of Biochemistry: Oligomycin
- Spektrum.de, Lexicon of Biology: Oligomycin
literature
- Liarisa A. Shchepina, Olga Y. Pletjushkina, Armine V. Avetisyan, Liora E. Bakeeva, Elena K. Fetisova, Denis S. Izyumov, Valeria B. Saprunova, Mikhail Y. Vyssokikh, Boris V. Chernyak, Vladimir P. Skulachev: Oligomycin, inhibitor of the F0 part of H + -ATP-synthase, suppresses the TNF-induced apoptosis. In: Oncogene . Volume 21, No. 53, pp. 8149-8157, November 2002, doi: 10.1038 / sj.onc.1206053 (free full text).
Individual evidence
- ↑ a b c d e f data sheet Oligomycin A ≥95% (HPLC) from Sigma-Aldrich , accessed on August 25, 2017 ( PDF ).
- ↑ Entry on Oligomycins. In: Römpp Online . Georg Thieme Verlag, accessed on August 25, 2017.
- ↑ Experimenting with isolated mitochondria: state III respiration. David R. Caprette, Rice University , accessed August 25, 2017 .
- ^ B. Chance, GR Williams: Respiratory enzymes in oxidative phosphorylation. III. The steady state . In: The Journal of Biological Chemistry . tape 217 , no. 1 , 1955, pp. 409-427 , PMID 13271404 (free full text).
- ^ Y. Kagawa, E. Racker: Partial resolution of the enzymes catalyzing oxidative phosphorylation. 8. Properties of a factor conferring oligomycin sensitivity on mitochondrial adenosine triphosphatase . In: The Journal of Biological Chemistry . tape 241 , no. 10 , 1966, pp. 2461-2466 , PMID 4223640 (free full text).
- ↑ Hans-Walter Heldt, Birgit Piechulla: Plant biochemistry . Springer Spectrum, Berlin / Heidelberg 2015, ISBN 978-3-662-44398-9 , pp. 118 , doi : 10.1007 / 978-3-662-44398-9 .
- ↑ M. Jastroch, AS Divakaruni, S. Mookerjee, JR Treberg, MD Brand: Mitochondrial proton and electron leaks . In: Essays in biochemistry . 47, No. 1, 2010, pp. 53-67. doi : 10.1042 / bse0470053 . PMID 20533900 . PMC 3122475 (free full text).
- ↑ M. Nakata, T. Ishiyama, S. Akamatsu, Y. Hirose, H. Maruoka, R. Suzuki, K. Tatsuta: Synthetic studies on oligomycins. Synthesis of the oligomycin B spiroketal and polypropionate portions . In: Bulletin of the Chemical Society of Japan . 68, No. 3, 1995, pp. 967-89. doi : 10.1246 / bcsj.68.967 .