Penciclovir
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
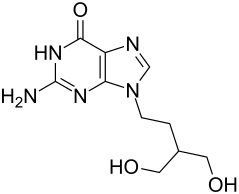
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Penciclovir | |||||||||||||||||||||
| other names |
2-Amino-9- [4-hydroxy-3-hydroxymethyl-butyl] -3,9-dihydropurin-6-one |
|||||||||||||||||||||
| Molecular formula | C 10 H 15 N 5 O 3 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class |
Antiviral |
|||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 253,26 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
275–277 ° C or 272–277 ° C (different morphology ) |
|||||||||||||||||||||
| solubility |
heavy in water (1.7 g l −1 at 20 ° C and pH 7) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Penciclovir is an analogue of the nucleobase guanine . It is used as an antiviral agent against herpes simplex viruses .
application
In Germany, penciclovir is approved for topical (external) use in herpes labialis and, like acyclovir, inhibits DNA synthesis in virus-infected cells. In contrast to the acyclovir known since 1977, Novartis holds the patent for this active ingredient. Inexpensive generics do not yet exist. Penciclovir, acyclovir and ganciclovir , as derivatives of the nucleobase guanine, have a very similar structure and effectiveness ( analogues ). Like acyclovir, penciclovir is effective both in the early phase and in the vesicle phase and shortens the healing process by up to 30% compared to the use of a placebo.
Trade names
Famvir (A), FeniVir (A, CH), Fenistil Pencivir (D). New since 2014 Pencivir (D).
Web links
- Chemotherapy Journal: Penciclovir
Individual evidence
- ↑ a b The Merck Index . An Encyclopaedia of Chemicals, Drugs and Biologicals . 14th edition, 2006, ISBN 978-0-911910-00-1 , p. 1222.
- ↑ a b Data sheet penciclovir from Sigma-Aldrich , accessed on October 22, 2016 ( PDF ). We are sorry, this page cannot be displayed .
- ↑ SL Spruance, R. Nett u. a .: Acyclovir cream for treatment of herpes simplex labialis: results of two randomized, double-blind, vehicle-controlled, multicenter clinical trials. In: Antimicrobial agents and chemotherapy . Volume 46, Number 7, July 2002, pp. 2238-2243, PMID 12069980 . PMC 127288 (free full text).
- ↑ a b G. W. Raborn, AY Martel u. a .: Effective treatment of herpes simplex labialis with penciclovir cream: combined results of two trials. In: Journal of the American Dental Association . Volume 133, Number 3, March 2002, pp. 303-309, PMID 11934185 .