Radical starter
Free radical starter is the name given to a specific group of chemical compounds that are useful for some chemical synthesis. They are initiators of radical reactions (see also: chemical reaction ) that arise through thermolytic (starting at room temperature) or photolytic cleavage. They thus form those reactive radicals which “start” the desired radical reaction (e.g. radical polymerization ). Preferred starters are azo compounds and peroxides . The radical initiator has an azo group (-N = N-), this is also known as azo initiators referred to.
Well-known radical starters
The formation of the radicals occurs through homolytic cleavage of a bond. Free radical initiators are indispensable in the manufacture of several economically important plastics (such as polyvinyl chloride or polystyrene ).
Azoisobutyronitrile (AIBN)
Azobis (isobutyronitrile) breaks down into two isobutyronitrile radicals and a nitrogen molecule:
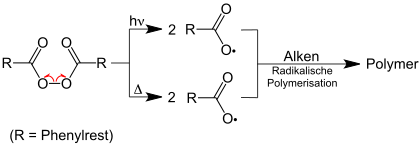
Dibenzoyl peroxide (DBPO)
Dibenzoyl peroxide DBPO breaks down into two benzoyloxy radicals, whereby the cleavage of the oxygen-oxygen bond can take place thermolytically or photolytically:
The formation of benzoyloxy radicals is a technically important radical initiation reaction in the production of polymers.
Inorganic peroxides
Inorganic peroxides such as peroxodisulfates decompose when heated to form sulfate radicals :
Polystyrene can be produced, for example, by initiation with peroxodisulfate.
Other
- Bis (2-ethylhexyl) peroxydicarbonate , e.g. B. vinyl chloride to PVC and for the crosslinking of z. B. polypropylene.
- Methyl ethyl ketone peroxide initiates the polymerization of unsaturated polyester resins . It is created by the reaction of butanone with hydrogen peroxide .
See also
Individual evidence
- ↑ Bernd Grunwald, Karl-Heinz Scharf: Elements chemistry. Teaching work for high schools . Klett , Stuttgart 1998, ISBN 3-12-759900-5 .
- ↑ Entry on butan-2-one. In: Römpp Online . Georg Thieme Verlag, accessed on November 01, 2016.