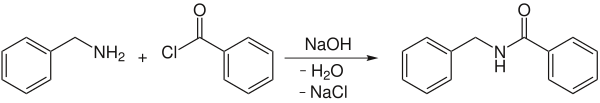Schotten-Baumann method
The Schotten-Baumann method is a name reaction from the field of organic chemistry that was first published in 1883 by the German chemists Carl Schotten (1853–1910) and Eugen Baumann (1846–1896). It comprises the reaction of amines , alcohols or phenols with carboxylic acid halides - mostly carboxylic acid chlorides - in the presence of at least stoichiometric amounts of aqueous alkali metal hydroxide solutions to give the corresponding amides or esters .
Overview
A carboxylic acid halide, here carboxylic acid chloride, is reacted with an alcohol to form an ester:
The reaction only takes place when an aqueous alkali metal hydroxide solution is added, in this case aqueous sodium hydroxide solution .
Alternatively, a carboxylic acid halide, here carboxylic acid chloride, can be converted to an amide by reaction with a primary or secondary amine.
The reaction only takes place when an aqueous alkali metal hydroxide solution is added, in this case aqueous sodium hydroxide solution .
Reaction mechanism
The following proposal for the reaction mechanism is based on the example of the reaction of a carboxylic acid chloride with a primary alcohol. The reaction process for all other variants mentioned in the Overview section is analogous:
The primary alcohol is added to the carboxylic acid chloride 1 . After the subsequent proton transfer , the hemiacetal 2 is formed . Ester 3 is formed by deprotonating the hydroxide group and splitting off the chlorine atom as a chloride ion .
Application examples
An application example is the reaction of benzylamine with benzoyl chloride to form N -benzoic acid amide:
According to the principle of the Schotten-Baumann reaction, the trihydric alcohol glycerol can also be converted into the diester by reacting with benzoyl chloride in the presence of sodium hydroxide solution , whereby the two primary hydroxyl groups are esterified, but the secondary hydroxyl group does not react.
Unicorn variant
In this variant of the Schotten-Baumann reaction, pyridine serves as a base and often also as a solvent . It initially reacts with the acid chlorides to form highly reactive pyridinium compounds . These are then attacked nucleophilically by alcohol or amines with the formation of esters or amides . The hydrogen chloride formed is bound as pyridine hydrochloride . In contrast to the Schotten-Baumann reaction, saponification of the ester is avoided here when working with dry alcohol. Pyridine acts as an acid binding agent.
The variant is named after its discoverer Alfred Einhorn .
Individual evidence
- ↑ Entry on Schotten-Baumann reaction. In: Römpp Online . Georg Thieme Verlag, accessed on June 15, 2014.
- ↑ a b c d e f g h i László Kürti and Barbara Czakó .: Strategic Applications of Named Reactions in Organic Synthesis: Background and Detailed Mechanisms , Elsevier Academic Press, 2005, pp. 398–399, ISBN 978-0-12- 429785-2 .
- ↑ a b M. B. Smith, J. March: March's Advanced Chemistry , 5th Edition, John Wiley and Sons, New York, 2001 , pp. 482-506.
- ↑ H. Hauptmann, University of Regensburg: Exercises for the lecture Organic Chemistry II for students of teaching and biology (PDF; 26 kB).
- ↑ Klaus Schwetlick: Organikum , 23rd completely revised and updated edition, Wiley-VCH, Weinheim 2009, ISBN 978-3-527-32292-3 .