Succinonitrile
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Succinonitrile | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 4 H 4 N 2 | |||||||||||||||
| Brief description |
colorless waxy or beige solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 80.09 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
|
|||||||||||||||
| Melting point |
|
|||||||||||||||
| boiling point |
|
|||||||||||||||
| Vapor pressure |
|
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.4173 (60 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Succinonitrile (succinic acid dinitrile ) is a water-soluble α, ω- dinitrile that is derived from succinic acid, which has recently attracted greater interest as a platform chemical made from renewable raw materials .
Bio-based succinonitrile can also be obtained from the amino acids L - glutamic acid and L - glutamine and could serve as a raw material for the potential polyamide building block putrescine .
Manufacturing
The first synthesis of succinonitrile (referred to here as cyanoethylene) was reported as early as 1861. The reaction of 1,2-dibromoethane with potassium cyanide in ethanol in the sense of a Kolbe nitrile synthesis produces succinonitrile.
A yield of 50% is given for this reaction in the organic matter specification.
The reaction of hydrogen cyanide with acrylonitrile in the presence of the samarium complex Cp * 2 Sm (thf) 2 [(pentamethylcyclopentadienyl) 2 Sm (tetrahydrofuran) 2 ], also known as decamethylsamarocene, leads to succinonitrile in 89% yield.
The reaction of gaseous ethylene oxide and hydrogen cyanide at 350–400 ° C on a silica gel contact also produces succinonitrile in modest yields.
By ammoxidation of 1,3-butadiene in a titanium - tungsten -contact also succinonitrile to be obtained.
The standard industrial route to succinonitrile is the addition of hydrogen cyanide to acrylonitrile, e.g. B. in the presence of alkaline media or trialkylamines such as triethylamine ,
very good yields of up to 95% and purities of up to 99.5% can be achieved under relatively mild reaction conditions (around 60 ° C). The addition of N , N -dimethylformamide or N , N -dimethylacetamide shortens the reaction time to about 60 minutes with comparable yields. The process can also be carried out continuously.
Succinic acid , e.g. B. also in aqueous solution as a result of a fermentative production, can be converted to succinonitrile in a contact of silicon orthophosphate Si 3 (PO 4 ) 4 at 350-425 ° C with ammonia via the intermediately formed diamide in 70% yield.
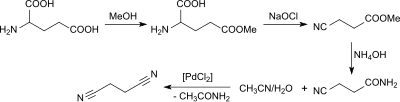
Succinonitrile can be obtained from glutamine and preferably from glutamic acid of biogenic origin in a multi-stage synthesis via 5-methyl glutamic acid, 3-cyanopropionic acid and 3-cyanopropionic acid amide.
The 3-cyanopropionic acid amide is converted into the succinonitrile with aqueous acetonitrile in the presence of palladium (II) chloride .
properties
Succinonitrile is an odorless, waxy, white, water-soluble solid that is present as a plastic crystal at room temperature and shows a solid-solid phase transition at 238 K.
In the early literature succinonitrile is described as non-distillable and below 37 ° C as a “light brown crystalline mass”, above this temperature as an oily liquid with a sharp, unpleasant taste. From dilute solutions, e.g. B. in acetone, succinonitrile shows beautiful dendritic crystal growth.
Applications
As an additive in solid polymer electrolytes , succinonitrile increases the ionic conductivity and improves the mechanical properties of solid electrolyte - lithium-ion accumulators .
Succinonitrile is also suitable as a starting compound for 2-pyrrolidone , with succinonitrile being partially hydrogenated with Raney nickel to give 3-aminopropionitrile in the first stage and then hydrolyzed with water under pressure at 210 ° C. to give γ-butyrolactam.
Complete hydrogenation of succinonitrile provides 1,4-diaminobutane , as the α, ω- diamine - monomer for polyamides , z. B. polyamide 4.6 is possible.
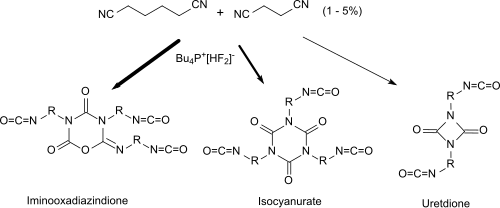
Succinonitrile is suitable as an additive when reacting with monomeric di- and / or tri- isocyanates (1 - 5% based on the diisocyanate hexamethylene diisocyanate HDI) in the presence of fluorides as catalysts for the production of polyisocyanates with high proportions of iminooxadiazinedione groups (asymmetric isocyanate trimers) . The polyisocyanates obtained have reactive free isocyanate groups and can serve as isocyanate components for polyurethane plastics .
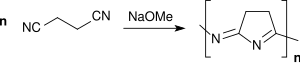
As a bifunctional monomer, succinonitrile can be converted to poly (1-pyrrolin-2-yl-5-ylidenenitrilo), a semiconductor soluble in NaOH and DMSO, by anionic polymerization with the catalyst sodium methoxide in methanol
and with the catalyst potassium tert butoxide in substance contrast to poly [(4-amino-2.5.6-pyrimidintriyl) -2.6-dimethylene], a soluble in DMSO non-conductors , are polymerized.
Individual evidence
- ↑ a b c d e f g h Data sheet Succinonitrile from Sigma-Aldrich , accessed on January 15, 2016 ( PDF ).
- ↑ a b Datasheet Succinonitrile at Acros, accessed on January 15, 2016.
- ↑ a b c d e f Lide, DR (ed.), CRC Handbook of Chemistry and Physics, 81st Edition, CRC Press LLC, Boca Raton, FL 2000, p. 3-92.
- ↑ a b c d e f Entry on succinonitrile in the GESTIS substance database of the IFA , accessed on January 9, 2019(JavaScript required) .
- ↑ a b c d Entry on succinonitriles at TCI Europe, accessed on January 15, 2016.
- ↑ S. Vaswani, PEP Review 2010-14, Bio-based Succinic Acid, SRI Consulting, 2014. (PDF; 2.5MB)
- ↑ a b T.M. Lammen, J. Le Notre, MCR Franssen, EL Scott, JPM Sanders: Synthesis of biobased succinonitrile from glutamic acid and glutamine . In: Chemsuschem . tape 4 , no. 6 , 2011, p. 785-791 , doi : 10.1002 / cssc.201100030 .
- ↑ a b M. Simpson: About Cyanäthylen and succinic acid . In: Justus Liebigs Ann. Chem. Band 118 , no. 3 , 1861, p. 373-376 , doi : 10.1002 / jlac.18611180317 .
- ↑ M. Simpson: About the synthesis of succinic acid and pyrowartaric acid . In: Justus Liebigs Ann. Chem. Band 121 , no. 2 , 1862, p. 153-165 , doi : 10.1002 / jlac.18621210203 .
- ^ Klaus Schwetlick: Organikum - organic-chemical basic internship . 24th edition. Wiley-VCH, Weinheim 2015, ISBN 978-3-527-33968-6 .
- ^ WJ Evans, I. Bloom, WE Hunter, JL Atwood: Synthesis and x-ray crystal structure of a soluble divalent organosamarium complex . In: J. Am. Chem. Soc. tape 103 , no. 21 , 1981, p. 6507-6508 , doi : 10.1021 / ja00411a046 .
- ↑ Y. Kawasaki, A. Fujii, Y. Nakano, S. Sakaguchi, Y. Ishii: Acetylcyanation of aldehydes with acetone cyanohydrin and isoprenylacetate by Cp * 2Sm (thf) 2 . In: J. Org. Chem. Band 64 , no. 1 , 1999, p. 4214-4216 , doi : 10.1021 / jo990030o .
- ↑ Patent US2427601 : Production of organic nitriles. Applied March 30, 1945 , published September 16, 1947 , applicant: EI Du Pont de Nemours and Company, inventor: CR Harris.
- ↑ Patent US20080004462A1 : Catalyst for the preparation of fumaronitrile and / or maleonitrile. Filed November 21, 2006 , published January 3, 2008 , inventor: AV Peters, PAC Schevelier.
- ↑ Patent DE707852 : Process for the production of nitriles. Registered on April 21, 1938 , published on May 29, 1941 , applicant: IG Farbenindustrie AG, inventor: P. Kurtz.
- ↑ Patent US2434606 : Method of preparing succinonitrile. Filed August 18, 1945 , published January 13, 1948 , applicant: American Cyanamid Co., inventor: EL Carpenter.
- ↑ Patent EP0016482A1 : Process for the preparation of succinonitrile. Registered on February 20, 1980 , published on October 1, 1980 , applicant: Stamicarbon BV, inventor: GH Suverkropp, JGM Nieuwkamp.
- ↑ Patent US7371884B2 : Process for preparing succinonitrile and use of succinonitrile. Registered on December 3, 2002 , published on May 13, 2008 , applicant: DSM IP Assets BV, inventor: H. Oevering, FHAM Vandenbooren, O. Poorter.
- ↑ Patent US2698337 : Hydrocyanation. Applied on August 23, 1951 , published December 28, 1954 , Applicant: Monsanto Chemical Co., Inventor: RL Heider, HM Walker.
- ↑ Patent US2842584 : Production of succinonitrile. Applied December 11, 1956 , published July 8, 1958 , Applicant: Carbogen Corp., Inventor: LJ Christmann.
- ↑ Patent US20110288324A1 : Method for manufacturing compounds including nitrile functions. Filed November 26, 2009 , published November 24, 2011 , inventors: R. Jacquot, P. Marion.
- ^ SI Maffioli, E. Marzorati, A. Marazzi: Mild and reversible dehydration of primary amides with PdCl 2 in aqueous acetonitrile . In: Org. Lett. tape 7 , no. 23 , 2005, pp. 5237-5239 , doi : 10.1021 / ol052100l .
- ^ DV Nickel, SP Delaney, H. Bian, J. Zheng, TM Korter, DM Middleman: Terahertz Vibrational Modes of the Rigid Crystal Phase of Succinonitrile . In: J. Phys. Chem. Band 118 , no. 13 , 2014, p. 2442-2446 , doi : 10.1021 / jp411865n ( PDF; 1.5MB ).
- ↑ Science Simulations: succinonitrile Solidification of. In: youtube.com. March 30, 2011, accessed February 2, 2016 .
- ↑ A. Abouimrane, IJ Davidson: Solid Electrolyte Based on Succinonitrile and LiBOB - Interface Stability and Application in Lithium Batteries . In: J. Electrochem. Soc. tape 154 , no. 11 , 2007, p. A1021 – A1034 , doi : 10.1149 / 1.2781305 ( PDF; 445kB ).
- ↑ L.-Z. Fan, Y.-S. Hu, AJ Bhattacharyya, J. Maier: Succinonitrile as a Versatile Additive for Polymer Electrolytes . In: Adv. Funct. Mater. tape 17 , no. 15 , 2007, p. 2800–2807 , doi : 10.1002 / adfm.200601070 .
- ↑ P.-J. Alarco, Y. Abu-Lebdeh, A. Abouimrane, M. Armand: The plastic-crystalline phase of succinonitrile as a universal matrix for solid-state ionic conductors . In: Nature Mater. tape 3 , no. 7 , 2004, p. 476-481 , doi : 10.1038 / nmat1158 .
- ↑ Patent US3644402 : Process for producing α-pyrrolidone. Applied on May 6, 1969 , published on February 22, 1972 , Applicants: Sumitomo Chemical Co., Ltd., Inventors: K. Takagi, T. Matsuda, M. Murakami.
- ↑ Patent US4123438 : Process for preparing 2-pyrrolidones. Applied on September 1, 1977 , published October 31, 1978 , applicant: Stamicarbon BV, inventor: LH Geurts, PJN Meijer.
- ↑ Patent EP0022292 : Process for the preparation of a 2-pyrrolidone. Applied on June 28, 1980 , published on May 11, 1983 , applicant: Stamicarbon BV, inventor: HCJ de Man, A. Corvers, PJH Thomissen.
- ↑ Hans-Jürgen Arpe: Industrial organic chemistry . 6th edition. Wiley-VCH, Weinheim 2007, ISBN 978-3-527-31540-6 , pp. 113 .
- ↑ Patent US5254738 : Preparation of 1,4-alkylenediamines. Applied on February 19, 1991 , published October 16, 1993 , applicant: BASF AG, inventor: U. Koehler, H. Siegel, M. Irgang.
- ↑ Patent WO2015011068A1 : Use of succinonitrile in the production of polyisocyanates containing iminooxadiazinedione groups. Registered on July 21, 2014 , published on January 29, 2015 , applicant: Bayer Material Science AG, inventor: F. Richter, R. Halpaap.
- ↑ D. Wöhrle: Polymers from nitriles. I. Anionic polymerization of succinic acid dinitrile . In: Makromol. Chem. Band 160 , no. 1 , 1972, p. 83-97 , doi : 10.1002 / macp.1972.021600106 .