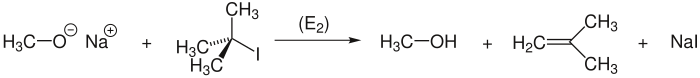Williamson ether synthesis
The Williamson synthesis (see also ether synthesis ) is used to produce symmetrical and asymmetrical ethers . It was developed in the 19th century (approx. 1854) by its namesake Alexander W. Williamson . The Williamson synthesis is a special case of the nucleophilic substitution (S N ), in which the nucleophile is an alkoxide (alkyl-O - , aryl-O - , etc.) is used.
The reaction mechanism corresponds - depending on the structure of the radicals R 1 and R 2 and the reaction conditions - to an S N 1 or S N 2 reaction:
The Williamson ether synthesis is technically a two-step process. First of all, an alcoholate has to be produced from the alcohol component, usually by reacting the alcohol with elemental sodium or potassium . Alternatively, the corresponding hydrides sodium hydride or potassium hydride can also be used. The latter variant has the advantage that the hydrides are more stable in moist air and easier to weigh than powdery substances.
In a second step, the alcoholate is reacted with the electrophile. The alkyl chlorides , alkyl bromides or alkyl iodides are frequently used as electrophiles ; sulfonic acid esters such as those of p -toluenesulfonic acid or methanesulfonic acid are also common.
A suitable solvent for the reaction is either the alcohol itself or another polar solvent such as dimethyl sulfoxide (DMSO), dimethylformamide (DMF) or hexamethylphosphoric acid triamide (HMPT).
A special case of Williamson's ether synthesis is the use of silver (I) oxide to etherify alcohols (in the example 4-hydroxy-2-butanone) 1 and alkyl halides (in the example benzyl bromide ) 2 .
Elimination instead of substitution
The Williamson ether synthesis does not usually succeed with tertiary haloalkanes . When trying them with alcoholates react to ethers, a is carried out instead of the Williamson synthesis (= substitution) elimination to form an alkene . For example, the reaction of 2-iodo-2-methylpropane with sodium methoxide produces 2-methyl-prop-1-ene ( isobutene ) and not the ether:
literature
- Organikum, 16th edition, VEB Deutscher Verlag der Wissenschaften Berlin 1985, p. 198, ISBN 3-326-00076-6 .
Individual evidence
- ^ Henry M. Leicester, Herbert S. Klickstein: Theory of Aetherification . In: Philosophical Magazine 1850 , 37 , 350–356.
- ↑ Masato Tanabe, Richard H. Peters: (R, S) -Mevalonolactone-2- 13 C In: Organic Syntheses . 60, 1981, p. 92, doi : 10.15227 / orgsyn.060.0092 ; Coll. Vol. 7, 1990, p. 386 ( PDF ).