Tungsten (II) chloride
| Crystal structure | |||||||
|---|---|---|---|---|---|---|---|
|
|||||||
| __ W 2+ __ Cl - | |||||||
| General | |||||||
| Surname | Tungsten (II) chloride | ||||||
| other names |
Tungsten dichloride |
||||||
| Ratio formula | WCl 2 | ||||||
| Brief description |
light gray solid |
||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 254.74 g mol −1 | ||||||
| Physical state |
firmly |
||||||
| density |
5.44 g cm −3 |
||||||
| Melting point |
500 ° C (decomposition) |
||||||
| solubility |
slightly soluble in water |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Tungsten (II) chloride is an inorganic chemical compound of tungsten from the group of chlorides .
Extraction and presentation
Tungsten (II) chloride can be obtained by reduction or disproportionation of tungsten (IV) chloride are obtained at 450-500 ° C.
It can also be obtained by reducing tungsten (VI) chloride with tungsten, hydrogen or sodium .
properties
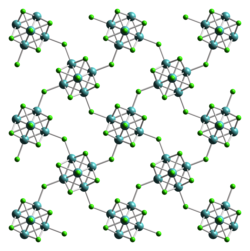
Tungsten (II) chloride is a light gray, crystalline, diamagnetic powder. Its crystal structure is isotypic to that of molybdenum (II) chloride in the space group Bbem (space group no. 64, position 5) with the lattice parameters a = 1127 pm, b = 1128 pm, c = 1404 pm. The structure contains an octahedral W 6 cluster and thus belongs to the metal clusters .
use
Tungsten (II) chloride is used as a starting material for a number of organometallic and inorganic syntheses.
Individual evidence
- ↑ a b c d Georg Brauer (Ed.) U. a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume III, Ferdinand Enke, Stuttgart 1981, ISBN 3-432-87823-0 , p. 1555.
- ↑ a b c W. M. Haynes (Ed.): CRC handbook of chemistry and physics. A ready-reference book of chemical and physical data . founded by David R. Lide. 93rd edition. CRC Press, Boca Raton 2012, ISBN 978-1-4398-8049-4 , pp. 4–96 (English, limited preview in Google Book Search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ A b c Leonard V. Interrante: Inorganic Syntheses, Nonmolecular Solids . John Wiley & Sons, 2009, ISBN 0-470-13296-5 , pp. 1 ( limited preview in Google Book search).
- ↑ The former name of this group of rooms was Bbam .
- ^ Karl-Heinz Lautenschläger: Pocket book of chemistry - Karl-Heinz Lautenschläger . Harri Deutsch Verlag, 2007, ISBN 978-3-8171-1761-1 , pp. 556 ( limited preview in Google Book search).
![{\ displaystyle \ mathrm {18 \ WCl_ {4} \ longrightarrow [W_ {6} Cl_ {8}] Cl_ {4} +12 \ WCl_ {5}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/e400b729ddcebccc39e45ca3bca3d2bd619bf330)
