Ziegler-Natta process
The Ziegler-Natta process (formerly also the Mülheim process ) describes a process for the production of polyolefins via coordinative insertion polymerization with organometallic catalysts. This process finds technical application in the synthesis of polyethylene and polypropylene .
history

Since 1938, polyethylene has been produced radically at high pressures and temperatures of up to 300 MPa and 300 ° C. In 1953 Karl Ziegler discovered at the Max Planck Institute for Coal Research in Mülheim an der Ruhr that ethene can be polymerized to polyethylene with titanium catalysts even at low pressures or later even at normal pressure and room temperature. The previously available polyethylene had different mechanical properties than the new, catalytically produced, linear polyethylene due to the radical polymerization and the resulting long chain branches. This had a higher crystallinity and thus a higher hardness and rigidity and thus opened up new fields of application for polyethylene. Just a few months after its discovery, the process was used industrially.
However, the initially low activity of the catalyst resulted in a large amount of catalyst remaining in the product, which therefore had to be laboriously processed. By continuously improving the catalyst activity, one gram of titanium in the catalyst can now produce well over 1000 kg of polymer and the catalyst residue remains in the product. The second namesake of the process is Giulio Natta , who successfully transferred transition metal catalysis to the polymerization of propene. Both were jointly awarded the Nobel Prize in Chemistry in 1963 for discoveries in the field of chemistry and technology of high polymers .
In 2007, the total volume of polypropylene using the Ziegler-Natta process was 45.1 million tons, and that of HDPE over 30 million tons. The polymers are used in the manufacture of pipes, gas lines, oil tanks, packaging materials and many other areas.
Ziegler-Natta catalysts
Classic catalysts
The catalytically active systems are organometallic mixed catalysts comprising an organometallic main group compound of the groups I, II or III (z. B. triethylaluminum ) and a transition metal compound, mainly of the groups IV to VI (z. B. titanium tetrachloride ).
These are also known as classic Ziegler-Natta catalysts (ZN catalysts). These are homogeneous or heterogeneous multicenter (multiple-site) catalysts. These are of enormous economic importance, especially for the production of polypropylene. Modern Ziegler-Natta catalysts are made from magnesium chloride , titanium tetrachloride , triethylaluminum and internal and external donors and achieve a conversion of 150 kg of polymer per gram of titanium. The name Ziegler catalysts goes back to a suggestion by the Italian chemist Giulio Natta, who for the first time succeeded in polymerizing propene in a stereospecific manner with the help of the mixed organometallic catalysts developed by Karl Ziegler .
Kaminsky catalysts
In addition to the classic, heterogeneous, multiple-site ZN catalysts, homogeneous, single-site catalysts have also recently been used for the commercial production of polyethylene and polypropylene. These are group 4 metallocene compounds in conjunction with the co-catalyst methylaluminoxane , so-called Kaminsky catalysts . Zirconocene complexes, which have a significantly higher catalytic activity than the corresponding titanocene complexes or hafnium systems, are of greatest importance. For procedural reasons, such homogeneous systems are nevertheless applied to solid, porous carrier particles.
mechanism
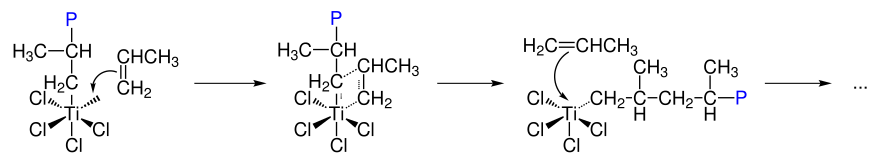
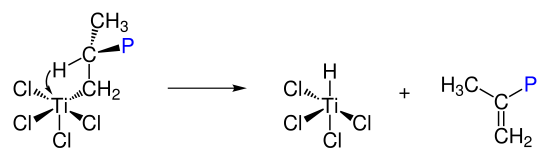
Ziegler-Natta catalysis is understood to be the coordinative insertion polymerization of olefins on Lewis acidic metal complexes. The catalyst systems generally consist of an organometallic main group component, such as triethylaluminum , and an organometallic transition metal component of subgroups four to eight.
In the first step, an octahedral complex is formed which has a free coordination site. Propene binds to the free coordination site and then inserts into the metal-carbon bond, which leads to the structure of the polymer.
Low pressure process
The low pressure process for polyethylene production is carried out in a paraffin oil in which the catalyst is dispersed. At normal pressure , but more often at ethene pressures of 2 to 8 bar, the reaction takes place in a stirred tank . When using conventional catalysts, the proportion of catalyst in the end product was high and a separation step had to be introduced for processing reasons. For this purpose, the catalyst was z. B. converted into soluble compounds with alcohols and separated.
Web links
- Nobel lecture by Karl Ziegler , December 12, 1963 (PDF file; 618 kB)
Individual evidence
- ↑ Ludwig L. Böhm: The ethylene polymerization with Ziegler catalysts 50 years after the discovery. In: Angewandte Chemie. 115, 2003, pp. 5162-5183, doi : 10.1002 / anie.200300580 .
- ↑ Rolf Mühlhaupt: Catalytic Polymerization and Post Polymerization Catalysis Fifty Years After the Discovery of Ziegler's Catalysts. In: Macromolecular Chemistry and Physics. 204, pp. 289-327, doi : 10.1002 / macp.200290085 .
- ^ MD Lechner, K. Gehrke and EH Nordmeier: Makromolekulare Chemie , 4th edition, Birkhäuser Verlag, 2010, pp. 91-96, ISBN 978-3-7643-8890-4 .
literature
- K. Ziegler, E. Holzkamp, H. Breil and H. Martin: The Mülheim normal pressure polyethylene process. In: Angewandte Chemie. 67, 1955, pp. 541-547, doi : 10.1002 / anie.19550671902 .
- G. Wilke, Angew. Chem., 75 ( 1963 ), p. 10.
- G. Natta, Angew. Chem., 76 , p. 553 ( 1964 ).