From Wikipedia, the free encyclopedia
Liquiritigenin
Names
IUPAC name
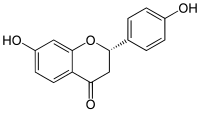
(2S )-4′,7-Dihydroxyflavan-4-one
Systematic IUPAC name
(2S )-7-Hydroxy-2-(4-hydroxyphenyl)-2,3-dihydro-4H -1-benzopyran-4-one
Identifiers
ChEBI
ChEMBL
ChemSpider
KEGG
UNII
InChI=1S/C15H12O4/c16-10-3-1-9(2-4-10)14-8-13(18)12-6-5-11(17)7-15(12)19-14/h1-7,14,16-17H,8H2/t14-/m0/s1
Key: FURUXTVZLHCCNA-AWEZNQCLSA-N
InChI=1/C15H12O4/c16-10-3-1-9(2-4-10)14-8-13(18)12-6-5-11(17)7-15(12)19-14/h1-7,14,16-17H,8H2/t14-/m0/s1
Key: FURUXTVZLHCCNA-AWEZNQCLBC
O=C2c3c(O[C@H](c1ccc(O)cc1)C2)cc(O)cc3
Properties
C 15 H 12 O 4
Molar mass
−1
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
Chemical compound
Liquiritigenin is a flavanone that was isolated from Glycyrrhiza uralensis Glycyrrhiza genus, including Glycyrrhiza glabra [1] estrogenic compound which acts as a selective agonist of the ERβ subtype of the estrogen receptor (ER),[2] ERα partial agonist at sufficient concentrations.[3] choleretic effect.[1]
Liquiritigenin,NADPH:oxygen oxidoreductase (hydroxylating, aryl migration) is an enzyme that uses liquiritigenin, O2 , NADPH and H+ to produce 2,7,4'-trihydroxyisoflavanone , H2 O, and NADP+ .
See also [ edit ] References [ edit ]
^ a b Kim, YW; Kang, HE; Lee, MG; Hwang, SJ; Kim, SC; Lee, CH; Kim, SG (2009). "Liquiritigenin, a flavonoid aglycone from licorice, has a choleretic effect and the ability to induce hepatic transporters and phase-II enzymes". American Journal of Physiology. Gastrointestinal and Liver Physiology . 296 (2): G372–81. doi :10.1152/ajpgi.90524.2008 . PMID 19074639 . ^ Mersereau, Jennifer E.; Levy, Nitzan; Staub, Richard E.; Baggett, Scott; Zogric, Tetjana; Chow, Sylvia; Ricke, William A.; Tagliaferri, Mary; et al. (2008). "Liquiritigenin is a plant-derived highly selective estrogen receptor β agonist" . Molecular and Cellular Endocrinology . 283 (1–2): 49–57. doi :10.1016/j.mce.2007.11.020 . PMC 2277338 PMID 18177995 . ^ Green, Sarah E (2015), In Vitro Comparison of Estrogenic Activities of Popular Women's Health Botanicals the original on 2016-02-22, retrieved 2016-01-01
ER Tooltip Estrogen receptor
Agonists
Steroidal: 2-Hydroxyestradiol 2-Hydroxyestrone 3-Methyl-19-methyleneandrosta-3,5-dien-17β-ol 3α-Androstanediol 3α,5α-Dihydrolevonorgestrel 3β,5α-Dihydrolevonorgestrel 3α-Hydroxytibolone 3β-Hydroxytibolone 3β-Androstanediol 4-Androstenediol 4-Androstenedione 4-Fluoroestradiol 4-Hydroxyestradiol 4-Hydroxyestrone 4-Methoxyestradiol 4-Methoxyestrone 5-Androstenediol 7-Oxo-DHEA 7α-Hydroxy-DHEA 7α-Methylestradiol 7β-Hydroxyepiandrosterone 8,9-Dehydroestradiol 8,9-Dehydroestrone 8β-VE2 10β,17β-Dihydroxyestra-1,4-dien-3-one (DHED) 11β-Chloromethylestradiol 11β-Methoxyestradiol 15α-Hydroxyestradiol 16-Ketoestradiol 16-Ketoestrone 16α-Fluoroestradiol 16α-Hydroxy-DHEA 16α-Hydroxyestrone 16α-Iodoestradiol 16α-LE2 16β-Hydroxyestrone 16β,17α-Epiestriol (16β-hydroxy-17α-estradiol) 17α-Estradiol (alfatradiol )17α-Dihydroequilenin 17α-Dihydroequilin 17α-Epiestriol (16α-hydroxy-17α-estradiol) 17α-Ethynyl-3α-androstanediol 17α-Ethynyl-3β-androstanediol 17β-Dihydroequilenin 17β-Dihydroequilin 17β-Methyl-17α-dihydroequilenin Abiraterone Abiraterone acetate Alestramustine Almestrone Anabolic steroids (e.g., testosterone and esters , methyltestosterone , metandienone (methandrostenolone) , nandrolone and esters , many others; via estrogenic metabolites)Atrimustine Bolandiol Bolandiol dipropionate Butolame Clomestrone Cloxestradiol
Conjugated estriol Conjugated estrogens Cyclodiol Cyclotriol DHEA DHEA-S ent -EstradiolEpiestriol (16β-epiestriol, 16β-hydroxy-17β-estradiol) Epimestrol Equilenin Equilin ERA-63 (ORG-37663) Esterified estrogens Estetrol Estradiol
Estramustine Estramustine phosphate Estrapronicate Estrazinol Estriol
Estrofurate Estrogenic substances Estromustine Estrone
Etamestrol (eptamestrol) Ethinylandrostenediol
Ethinylestradiol
Ethinylestriol Ethylestradiol Etynodiol Etynodiol diacetate Hexolame Hippulin Hydroxyestrone diacetate Lynestrenol Lynestrenol phenylpropionate Mestranol Methylestradiol Moxestrol Mytatrienediol Nilestriol Norethisterone Noretynodrel Orestrate Pentolame Prodiame Prolame Promestriene RU-16117 Quinestradol Quinestrol Tibolone Xenoestrogens: Anise -related (e.g., anethole , anol , dianethole , dianol , photoanethole )Chalconoids (e.g., isoliquiritigenin , phloretin , phlorizin (phloridzin) , wedelolactone )Coumestans (e.g., coumestrol , psoralidin )Flavonoids (incl. 7,8-DHF , 8-prenylnaringenin , apigenin , baicalein , baicalin , biochanin A , calycosin , catechin , daidzein , daidzin , ECG , EGCG , epicatechin , equol , formononetin , glabrene , glabridin , genistein , genistin , glycitein , kaempferol , liquiritigenin , mirificin , myricetin , naringenin , penduletin , pinocembrin , prunetin , puerarin , quercetin , tectoridin , tectorigenin )Lavender oil Lignans (e.g., enterodiol , enterolactone , nyasol (cis -hinokiresinol) )Metalloestrogens (e.g., cadmium )Pesticides (e.g., alternariol , dieldrin , endosulfan , fenarimol , HPTE , methiocarb , methoxychlor , triclocarban , triclosan )Phytosteroids (e.g., digitoxin (digitalis ), diosgenin , guggulsterone )Phytosterols (e.g., β-sitosterol , campesterol , stigmasterol )Resorcylic acid lactones (e.g., zearalanone , α-zearalenol , β-zearalenol , zearalenone , zeranol (α-zearalanol) , taleranol (teranol, β-zearalanol) )Steroid -like (e.g., deoxymiroestrol , miroestrol )Stilbenoids (e.g., resveratrol , rhaponticin )Synthetic xenoestrogens (e.g., alkylphenols , bisphenols (e.g., BPA , BPF , BPS ), DDT , parabens , PBBs , PHBA , phthalates , PCBs )Others (e.g., agnuside , rotundifuran ) MixedSERMs Tooltip Selective estrogen receptor modulators ) Antagonists
Coregulator-binding modulators: ERX-11
GPER Tooltip G protein-coupled estrogen receptor
Agonists Antagonists Unknown