Molecular sensor
A molecular sensor or chemosensor is a molecule that exhibits a detectable change when interacting with an analyte. Molecular sensors combine molecular recognition with some form of recording so that, for example, the presence of a guest can be observed in the case of a host molecule. The term supramolecular analytical chemistry has recently been used to describe the application of molecular sensors in analytical chemistry.
Earlier examples of molecular sensors are crown ethers with a high affinity for sodium ions, but not for potassium ions, and forms of metal recognition, so-called complexones: the traditional pH indicators are provided with functional groups so that they can detect metal ions. This receptor-spacer-reporter concept is discussed on the basis of photo-induced electron transfer (PET). An example of this is the sensor for heparin .

|
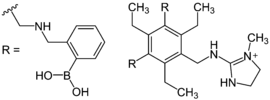
|

|
| Heparin sensor | Tannin sensor | Saxitoxin sensor |
There are receptors that are not specific for a molecule, but for a class of compounds. An example of this is the analysis of various tannins, such as tannin, which arise in old whiskey in oak barrels. You can see a correlation with the age of the whiskey. A similar receptor recognizes tartrates in wine.
The substance saxitoxin is a neurotoxin (nerve poison) found in shellfish and chemical weapons. A sensor was found for this substance, also based on PET. Interactions between the saxitoxin and the crown ether part of the sensor lead to the fluorescence quenching of the fluorophore in the sensor via a PET process , thus switching it on and off.
In another strategy, which is called indicator displacement assay (IDA), the analyte, such as a citrate or phosphate ion, replaces the fluorescent indicator in the indicator-host complex.
See also
Individual evidence
- ↑ Luminescent molecular sensors based on analyte coordination to transition-metal complexes Cerrie W. Rogers and Michael O. Wolf Coordination Chemistry Reviews; 2002 ; 233-24, pp. 341-350; (Review) doi : 10.1016 / S0010-8545 (02) 00023-1 .
- ↑ a b Eric V. Anslyn: Supramolecular Analytical Chemistry J. Org. Chem .; 2007 ; 72 (3), pp. 687-699; doi : 10.1021 / jo0617971 , PMID 17253783 .
- ↑ Design principles of fluorescent molecular sensors for cation recognition Bernard Valeur and Isabelle Leray Coordination Chemistry Reviews; 2000 ; 205, pp. 3-40; (Review) doi : 10.1016 / S0010-8545 (00) 00246-0 .
- ↑ Visible Fluorescence Chemosensor for Saxitoxin Robert E. Gawley, Hua Mao, M. Mahbubul Haque, John B. Thorne, and Jennifer S. Pharr J. Org. Chem .; 2007 ; 72 (6), pp. 2187-2191; (Article) doi : 10.1021 / jo062506r .
- ↑ Nguyen, B .; Anslyn, E. (2006). "Indicator-displacement assays". Coor. Chem. Rev. 250 (23-24): 3118-3127. doi : 10.1016 / j.ccr.2006.04.0 .
- ↑ Cattrall, RW (1997). Chemical sensors. Oxford University Press. ISBN 0-19-850090-4 .
