1-methylcytosine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
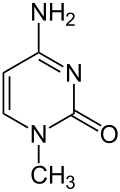
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 1-methylcytosine | |||||||||||||||
| other names |
4-amino-1-methyl-2 (1 H ) -pyrimidinone |
|||||||||||||||
| Molecular formula | C 5 H 7 N 3 O | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 125.13 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
1-methylcytosine is a heterocyclic organic compound with a pyrimidine backbone. It is a derivative of the nucleobase cytosine with an additional methyl group in position 1. It occurs as part of the Hachimoji DNA .
Extraction and presentation
1-Methylcytosine can be obtained by reacting cytosine with dimethylformamide-dimethylacetal in the presence of trifluoroacetic acid.
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ TJ Kistenmacher, M. Rossi: 1-Methylcytosine: a refinement . In: Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry . 33, No. 12, December 15, 1977, pp. 3962-3965. doi : 10.1107 / S0567740877012618 .
- ↑ Shuichi Hoshika: Hachimoji DNA and RNA: A genetic system with eight building blocks . In: Science . 363, No. 6429, February 22, 2019, pp. 884-887. doi : 10.1126 / science.aat0971 .
- ↑ Ramachandra S. Hosmane, Nelson J. Leonard: Simple Convenient Synthesis of 1-Methylcytosine. In: Synthesis . 1981, 1981, pp. 118-119, doi : 10.1055 / s-1981-29352 .