Ammonium perrhenate
| Crystal structure | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| __ N 3− __ H + __ Re 7+ __ O 2− | |||||||||||||||||||
| Space group |
I 4 1 / a (No. 88) |
||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Ammonium perrhenate | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Ratio formula | NH 4 ReO 4 | ||||||||||||||||||
| Brief description |
white solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 268.24 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
3.97 g cm −3 (25 ° C) |
||||||||||||||||||
| Melting point |
Decomposition from 200 ° C |
||||||||||||||||||
| solubility |
slightly soluble in water
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Ammonium perrhenate is an inorganic chemical compound from the group of perrhenates .
Extraction and presentation
Ammonium perrhenate can be obtained by reacting a perrhenic acid solution with ammonia .
properties
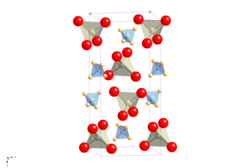
Ammonium perrhenate is a white solid that is sparingly soluble in water. It decomposes at 400 ° C into rhenium (IV) oxide , water and nitrogen . It has a tetragonal crystal structure of the Scheelite type with the space group I 4 1 / a (space group no. 88) and the lattice parameters a = 587.1 pm and c = 1294.2 pm.
use
The rhenium (III) organohydrazides formed when ammonium perrhenate is reduced with 2-hydrazinopyridine and triphenylphosphine are potential radiopharmaceuticals.
Individual evidence
- ↑ a b c d e data sheet Ammonium perrhenate, 99.999% trace metals basis from Sigma-Aldrich , accessed on August 2, 2013 ( PDF ).
- ↑ a b c Georg Brauer: Ammonium perrhenate . In: Handbook of Preparative Inorganic Chemistry . Ferdinand Enke Verlag, Stuttgart 1954, p. 1108 .
- ↑ a b O. Glemser: Ammonium Perrhenate . In: Handbook of Preparative Inorganic Chemistry , 2nd Ed. Edited by G. Brauer, Academic Press, 1963, New York. Volume 1, pp. 1484-1485.
- ↑ a b Entry on ammonium perrhenate in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ↑ a b Georg Brauer: Handbook of preparative inorganic chemistry . 3., reworked. Edition. tape III . Enke, Stuttgart 1981, ISBN 3-432-87823-0 , pp. 1633 .


