Glymphatic system
The glyphatic system is a presumed disposal system for waste materials in the central nervous system (CNS) of vertebrates , i.e. in the brain and spinal cord . The name is a neologism (a new word created) from the terms glia and lymphatic system and was introduced by a research group led by Maiken Nedergaard ( Rochester and Copenhagen ) in 2013, who first described the system as a functional unit.
Similar to the lymphatic system, which ends outside the meninges , i.e. does not occur in the CNS, the glymphatic system is understood as a flowing through-flow system for the removal of superfluous and harmful material. The transport fluid is released into the lymphatic system. Despite some skeptical comments, interest in its discovery has grown rapidly, particularly because of its role in sleep and neurodegenerative diseases such as Alzheimer's disease , Parkinson's disease, and amyotrophic lateral sclerosis (ALS).
Cycle
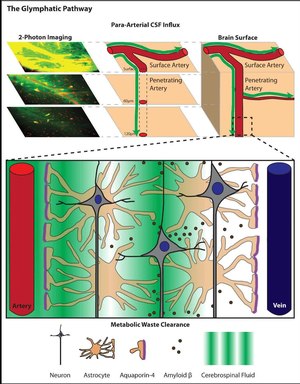
The arteries of the CNS have from their entry around by the meninges around its outer wall an additional, very narrow vascular space, a so-called perivascular space ( Spatium perivascular ) of the blood vessels in the CNS, the term Virchow-Robin space bears. A small part of the cerebrospinal fluid ( liquor cerebrospinalis ) from the space between the skull and the brain ( subarachnoid space or outer liquor space ) reaches all areas of the CNS in a constant current - driven by the wave movements of the arterial walls triggered by the pulse beat .
The transport space along the arteries is in turn tightly enclosed by appendages of the astrocytes (star cells), which make up the majority of the glial cells. They absorb the cerebrospinal fluid that flows by and pass it on to the entire cell space (interstitium) in the brain and spinal cord.
The fluid continuously enriched in this way in the interstitial space ( interstitial fluid ) seeps from the areas near the arteries across the tissue to the venous plexus , from where it again leaves the brain through perivascular spaces - this time along the outer walls of veins - and down to the meninges is fed into vessels of the lymphatic system. On the way through the tissue, waste materials are absorbed, which are then processed or disposed of in the liver and kidneys after transport via the perivascular spaces, lymphatic system and finally the general blood circulation .
background
The blood-brain barrier , the blood-liquor barrier and the lock-out of the lymphatic system provide special protection for the CNS from the uncontrolled penetration of large molecules ( macromolecules ) and their internal penetration through vascular walls . Waste materials cannot therefore be disposed of here as in the rest of the body. The situation is exacerbated by the more intensive metabolism in the CNS. For this reason there have long been suspicions and indications of a special disposal system.
As early as 1968 it was assumed that the Virchow-Robin room could be a transport route corresponding to the lymphatic system. In 1985 this assumption was confirmed by the recording of the spread of special labeled proteins . The outflow of labeled proteins from the intercellular space was detected in 1988.
Detection methods
After the injection of fluorescently labeled and radiolabeled molecules of various sizes into the CSF of the cerebellomedullary cistern of mice, the entry, distribution and excretion of the substances in the brain were recorded in real time. This was achieved by scanning the top layer of the cortex through a closed observation window with two-photon microscopy at a depth of 60 and 120 μm. Large molecules, here fluorescein isothiocyanate – dextran-2000 (FITC-d2000) with a molecular mass of 2000 kilodaltons (kDa), did get into the Virchow-Robin space, but not into the interstitial space. Smaller molecules, here Alexa Fluor 594 hydrazide (A594) with 759 Da and Texas Red-Dextran-3 (TR-d3) with 3 kDa, on the other hand, also spread in the cell space, whereby the lighter A594 was significantly faster. The transport in deeper layers was proven by later histological examinations.
The active role of the astrocytes through the participation of their water channel aquaporin-4 (AQP4) at their contact points to the arteries was demonstrated by control experiments with transgenic mice lacking AQP4. With them, the influx of the labeled molecules was slowed and their leaching from the brain was reduced by about 70%. A little later, the circulatory function of the glyphatic system was also demonstrated in rats using contrast medium-based magnetic resonance imaging (MRT).
Mainly active in sleep
The comparison of the transport in awake and sleeping animals showed a decrease of about 95% in the awake state. It was also shown that the volume of the intercellular space increased during sleep due to the shrinkage of the cell bodies, with a share of the total volume of around 24% compared to around 14% in the waking state. During sleep there was therefore> 60% more space for fluid transport. Norepinephrine , a main modulator of wakefulness, has also proven to be a possible regulator of the volume of the intercellular space and thus of the effectiveness of the glymphatic system .
Protective functions
Since around 2008, it has become increasingly popular that protein misfolding diseases such as B. Alzheimer's are due to protein malformations not only inside the cell, but also in the space between cells. The importance of the glyphatic system for the removal of misfolded proteins from the brain has therefore been the subject of intensive research ever since. A connection is conceivable in all known neurodegenerative diseases .
Alzheimer
Deposits of beta-amyloids , so-called senile plaques , in the cell space are transported away by the glymphatic system . In mice, the removal of beta-amyloids was twice as fast during sleep as during wakefulness. This has been linked to the increased risk of those with insomnia of developing Alzheimer's disease. The increase in the risk of disease in old age was also associated with the corresponding, age-related decline in performance of the glymphatic system . Certain pathogenic forms of tau proteins characteristic of Alzheimer's disease are also transported away from the intercellular space by the glyphatic system .
Amyotrophic lateral sclerosis (ALS)
A hallmark of ALS is the accumulation of the misfolded enzyme SOD1 , and a 2015 hypothesis suggested that in ALS patients the function of the glymphatic system could be impaired and contribute to neurodegeneration.
Expanded perivascular spaces as a biomarker
Pathological expansions of perivascular spaces can be visualized in magnetic resonance imaging (MRI). There is evidence that such dilatation may indicate minor vascular damage, an increased risk of stroke , and the development of dementia . One object of research is whether biomarkers can be found in this way as early signs of neurodegenerative diseases .
literature
- JJ Iliff, AS Thrane, M. Nedergaard: The Glymphatic System and Brain Interstitial Fluid Homeostasis. In: Louis R. Caplan, José Biller, Megan C. Leary, Eng H. Lo, Ajith J Thomas, Midori Yenari, John H. Zhang (Eds.): Primer on Cerebrovascular Diseases. second edition. Academic Press, San Diego / USA 2017, ISBN 978-0-12-803059-2 , pp. 17-25. Google Books preview .
- P. Venkat, M. Chopp, J. Chen: New insights into coupling and uncoupling of cerebral blood flow and metabolism in the brain. In: Croatian medical journal. Volume 57, Number 3, June 2016, pp. 223-228. PMID 27374823 , PMC 4937223 (free full text) (review).
- J. Ramirez, C. Berezuk, AA McNeely, F. Gao, J. McLaurin, SE Black: Imaging the Perivascular Space as a Potential Biomarker of Neurovascular and Neurodegenerative Diseases. In: Cellular and molecular neurobiology. Volume 36, Number 2, March 2016, pp. 289-299, doi: 10.1007 / s10571-016-0343-6 . PMID 26993511 (Review), (PDF)
- K. Hitscherich, K. Smith, JA Cuoco, KE Ruvolo, JD Mancini, JR Leheste, G. Torres: The Glymphatic-Lymphatic Continuum: Opportunities for Osteopathic Manipulative Medicine. In: The Journal of the American Osteopathic Association. Volume 116, Number 3, March 2016, pp. 170–177, doi: 10.7556 / jaoa.2016.033 . PMID 26927910 (Review).
- NA Jessen, AS Munk, I. Lundgaard, M. Nedergaard: The Glymphatic System: A Beginner's Guide. In: Neurochemical research. Volume 40, number 12, December 2015, pp. 2583-2599, doi: 10.1007 / s11064-015-1581-6 . PMID 25947369 , PMC 4636982 (free full text) (review).
- T. Brinker, E. Stopa, J. Morrison, P. Klinge: A new look at cerebrospinal fluid circulation. In: Fluids and barriers of the CNS. Volume 11, 2014, pp. 10-25, doi: 10.1186 / 2045-8118-11-10 . PMID 24817998 , PMC 4016637 (free full text) (review).
- AR Mendelsohn, JW Larrick: Sleep facilitates clearance of metabolites from the brain: glymphatic function in aging and neurodegenerative diseases. In: Rejuvenation research. Volume 16, number 6, December 2013, pp. 518-523, doi: 10.1089 / rej.2013.1530 . PMID 24199995 (review).
Web links
- Maiken Nedergaard, Steven A. Goldman: Glymphatic System - Nocturnal Brainwashing. In: Spectrum of Science. 12-2016.
- Nora Schlueter: Nocturnal brainwashing. In: Image of Science. 18th October 2013.
- Sleep detoxifies the brain. In: Deutsches Ärzteblatt. 18th October 2013.
- Video: Short lecture by one of the participating researchers , 3:17 min, August 15, 2012.
Individual evidence
- ↑ a b c d N. A. Jessen, AS Munk, I. Lundgaard, M. Nedergaard: The Glymphatic System: A Beginner's Guide. In: Neurochemical research. Volume 40, number 12, December 2015, pp. 2583-2599, doi: 10.1007 / s11064-015-1581-6 . PMID 25947369 , PMC 4636982 (free full text) (review).
- ↑ D. Raper, A. Louveau, J. Kipnis: How Do Meningeal Lymphatic Vessels Drain the CNS? In: Trends in neurosciences. Volume 39, number 9, September 2016, pp. 581-586, doi: 10.1016 / j.tins.2016.07.001 . PMID 27460561 , PMC 5002390 (free full text) (review).
- ^ A b T. Brinker, E. Stopa, J. Morrison, P. Klinge: A new look at cerebrospinal fluid circulation. In: Fluids and barriers of the CNS. Volume 11, 2014, pp. 10-25, doi: 10.1186 / 2045-8118-11-10 . PMID 24817998 , PMC 4016637 (free full text) (review).
- ↑ JJ Iliff, M. Wang, Y. Liao, BA Plogg, W. Peng, GA Gundersen, H. Benveniste, GE Vates, R. Deane, SA Goldman, EA Nagelhus, M. Nedergaard: A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. In: Science Translational Medicine . Volume 4, number 147, August 2012, p. 147ra111, doi: 10.1126 / scitranslmed.3003748 . PMID 22896675 , PMC 3551275 (free full text).
- ↑ NN Haj-Yasein, GF Vindedal, M. Eilert-Olsen, GA Gundersen, .. Skare, P. Laake, A. Klungland, AE Thoren, JM Burkhardt, OP Ottersen, EA Nage: glial conditional deletion of aquaporin-4 (Aqp4) reduces blood-brain water uptake and confers barrier function on perivascular astrocyte endfeet. In: Proceedings of the National Academy of Sciences . Volume 108, Number 43, October 2011, pp. 17815-17820, doi: 10.1073 / pnas.1110655108 . PMID 21990350 , PMC 3203818 (free full text).
- ↑ JJ Iliff, H. Lee, M. Yu, T. Feng, J. Logan, M. Nedergaard, H. Benveniste: Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. In: The Journal of clinical investigation. Volume 123, number 3, March 2013, pp. 1299–1309, doi: 10.1172 / JCI67677 . PMID 23434588 , PMC 3582150 (free full text).
- ↑ L. Xie, H. Kang, Q. Xu, MJ Chen, Y. Liao, M. Thiyagarajan, J. O'Donnell, DJ Christensen, C. Nicholson, JJ Iliff, T. Takano, R. Deane, M. Nedergaard: Sleep drives metabolite clearance from the adult brain. In: Science. Volume 342, number 6156, October 2013, pp. 373–377, doi: 10.1126 / science.1241224 . PMID 24136970 , PMC 3880190 (free full text).
- ↑ AR Eugene, J. Masiak: The Neuroprotective Aspects of Sleep. In: MEDtube science. Volume 3, Number 1, March 2015, pp. 35-40. PMID 26594659 , PMC 4651462 (free full text) (review).
- ^ A. Schneider, M. Simons: Exosomes: vesicular carriers for intercellular communication in neurodegenerative disorders. In: Cell and tissue research. Volume 352, Number 1, April 2013, pp. 33-47, doi: 10.1007 / s00441-012-1428-2 . PMID 22610588 , PMC 3602607 (free full text) (review).
- ↑ JM Tarasoff-Conway, RO Carare, RS Osorio, L. Glodzik, T. Butler, E. Fieremans, L. Axel, H. Rusinek, C. Nicholson, BV Zlokovic, B. Frangione, K. Blennow, J. Ménard , H. Zetterberg, T. Wisniewski, MJ de Leon: Clearance systems in the brain-implications for Alzheimer's disease. In: Nature reviews. Neurology. Volume 11, number 8, August 2015, pp. 457-470, doi: 10.1038 / nrneurol.2015.119 . PMID 26195256 , PMC 4694579 (free full text) (review).
- ↑ JJ Iliff, MJ Chen, BA Plog, DM Zeppenfeld, M. Soltero, L. Yang, I. Singh, R. Deane, M. Nedergaard: Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. In: The Journal of neuroscience: the official journal of the Society for Neuroscience. Volume 34, number 49, December 2014, pp. 16180–16193, doi: 10.1523 / JNEUROSCI.3020-14.2014 . PMID 25471560 , PMC 4252540 (free full text).
- ^ RA Radford, M. Morsch, SL Rayner, NJ Cole, DL Pountney, RS Chung: The established and emerging roles of astrocytes and microglia in amyotrophic lateral sclerosis and frontotemporal dementia. In: Frontiers in cellular neuroscience. Volume 9, 2015, p. 414, doi: 10.3389 / fncel.2015.00414 . PMID 26578880 , PMC 4621294 (free full text) (review), especially picture 2.
- ↑ RM Kwee, TC Kwee: Virchow-Robin spaces at MR imaging. In: Radiographics: a review publication of the Radiological Society of North America, Inc. Volume 27, Number 4, 2007 Jul-Aug, pp. 1071-1086, doi: 10.1148 / rg.274065722 . PMID 17620468 (Review), (PDF) ( Memento of the original dated November 16, 2016 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ^ S. Groeschel, WK Chong, R. Surtees, F. Hanefeld: Virchow-Robin spaces on magnetic resonance images: normative data, their dilatation, and a review of the literature. In: Neuroradiology. Volume 48, Number 10, October 2006, pp. 745-754, doi: 10.1007 / s00234-006-0112-1 . PMID 16896908 (Review), (PDF)
- ↑ J. Ramirez, C. Berezuk, AA McNeely, F. Gao, J. McLaurin, SE Black: Imaging the Perivascular Space as a Potential Biomarker of Neurovascular and Neurodegenerative Diseases. In: Cellular and molecular neurobiology. Volume 36, Number 2, March 2016, pp. 289-299, doi: 10.1007 / s10571-016-0343-6 . PMID 26993511 (Review), (PDF)