Hydroxychloroquine
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
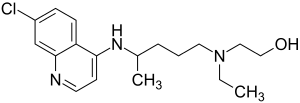
|
||||||||||||||||||||||
| Simplified structural formula without stereochemistry | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Hydroxychloroquine | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 18 H 26 ClN 3 O | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 335.87 g · mol -1 | |||||||||||||||||||||
| Melting point |
89-91 ° C |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Hydroxychloroquine is a drug analogous to chloroquine for the oral therapy of rheumatoid arthritis and collagenoses such as systemic lupus erythematosus and for the treatment and prevention of tropical malaria . Chemically it is structurally related to quinine .
pharmacology
Hydroxychloroquine inhibits heme - Polymerase the plasmodia and binds to DNA. It is absorbed into their vacuoles in plasmodia . Hydroxychloroquine inhibits the phase of life of the plasmodia in the erythrocytes . It is less toxic to the retina than chloroquine.
Chloroquine also acts as a zinc ionophore , thereby causing increased intracellular zinc concentrations. Zinc in turn has an inhibitory effect on the RNA polymerase of coronaviruses .
Like chloroquine, hydroxychloroquine inhibits autophagocytosis .
Hydroxychloroquine sulfate, a crystalline powder that is easily soluble in water , is used in pharmaceutical preparations .
Analytics
Similar to chloroquine, the substance can be qualitatively and quantitatively determined after appropriate sample preparation in the various test materials by coupling HPLC with mass spectrometry . The thin-layer chromatography and gas chromatography have been successfully used for the analysis.
Side effects
Chloroquine and hydroxychloroquine retinopathy can occur as a result of long-term use of chloroquine or hydroxychloroquine.
Severe hypoglycaemia , including cases of loss of consciousness, has occurred during treatment with hydroxychloroquine and can be life-threatening.
There have been reports of cardiomyopathies that can lead to heart failure , including cases with fatal outcome.
Hydroxychloroquine can lead to a dose-dependent prolongation of the QT interval . The risk of ventricular arrhythmias may be increased in patients with pre-existing cardiac diseases, or if substances that prolong the QT interval are used at the same time .
Therapeutic and experimental use
Rheumatic diseases
Hydroxychloroquine is approved for the treatment of rheumatoid arthritis and systemic lupus erythematosus (SLE), although the latency to the onset of action is relatively long and the effectiveness is less than that of methotrexate . Hydroxychloroquine is slightly better tolerated than chloroquine. In SLE, together with non-steroidal anti-inflammatory drugs, it is one of the first choice drugs . It is also used in the adjuvant treatment of juvenile idiopathic arthritis.
COVID-19
Chloroquine and hydroxychloroquine are being considered for treating COVID-19 . As before for chloroquine, in-vitro studies in cell culture showed an effectiveness of hydroxychloroquine against SARS-CoV-2 viruses at a mean effective concentration in the micromolar range.
A randomized controlled pilot study carried out in Shanghai with 30 patients could not find any advantage for the patients treated with hydroxychloroquine compared to the control group.
A clinical study from Marseille led by Didier Raoult came to positive results in the treatment of COVID-19 patients with hydroxychloroquine. This study has been criticized for its methodology. The virologist Christian Drosten stated that it did not allow any conclusions to be drawn about the actual effectiveness. The International Society of Antimicrobial Chemotherapy , in whose journal the study was published, distanced itself from the work on April 3, 2020. Didier Raoult subsequently became known worldwide as an advocate for hydroxychloroquine.
At the end of March 2020, the results of a randomized study were published in the previous month on 62 predominantly mildly ill patients at the Wuhan University Hospital . In the group treated with hydroxychloroquine, pneumonia improved within five days in 25 of 31 patients. In the untreated control group , there were 17 of 31 patients at the same time. 4 of the 62 patients who suffered a severe course after enrollment in the study were all in the control group. Two patients from the treated group experienced moderate side effects. In the treated group, a greater proportion of patients had a fever or cough when they entered the study. In addition, the treated group was slightly younger (44.1 vs. 45.2 years average age) and had a slightly higher proportion of women (54.9% vs. 51.7%). This could indicate a more advanced course of the disease, that the treated group per se was overall further along the course of the disease. The Federal Institute for Drugs and Medical Devices (BfArM) also considers the results of the first Chinese studies to be vulnerable.
At the same time, a great deal of public attention was directed to the use of hydroxychloroquine against COVID-19: US President Donald Trump expressed high expectations at a press conference and in a tweet on March 21, 2020. On April 5, 2020, he continued touting the drug, while Anthony Fauci , director of the National Institute of Allergy and Infectious Diseases , warned against jumping to conclusions. The governor of New York , Andrew Cuomo , announced a clinical trial that was to begin on 24 March 2020th An Arizona man who took a chloroquine-based product to treat fish due to a television report without medical supervision, died with symptoms of poisoning .
Chloroquine and hydroxychloroquine were recommended in the Belgian provisional treatment guidelines and the South Korean guidelines for COVID-19 patients.
In Germany, the BfArM approved a clinical phase III study with hydroxychloroquine as an investigational product on March 25, 2020 . Patients with mild or moderate COVID-19 disease should be included. In addition, information on off-label use was published in the context of individual healing attempts . Due to the intensive discussion of the potential suitability for the treatment of Covid-19, rheumatologists , represented for example by the European Rheumatism League (EULAR) , fear that supply shortages for rheumatoid patients will occur. The European Medicines Agency informed on 1 April 2020 that patients and healthcare professionals as well as hydroxychloroquine, chloroquine should use in approved applications, or at most within the framework of clinical trials or national emergency treatment programs for COVID-19th She warned that in addition to delivery bottlenecks, potentially serious side effects are possible. From April 3, 2020, pharmacies in Germany were temporarily only allowed to use drugs containing hydroxychloroquine for reasons of drug safety and to “ensure the care of chronically ill patients in the approved indications” if one of the approved indications was given and limited to the intended ones Dispense quantities. Even private prescriptions and sale to doctors for their own use were affected. The off-label use for Covid-19 was limited to hospitals. By April 10, the BfArM had approved four clinical studies with hydroxychloroquine. In two of these studies, people with mild COVID-19 are treated on an outpatient basis; the other two studies include moderately to severely ill, inpatients. In addition, a study by the World Health Organization in Germany is to be approved by the BfArM in mid-April, in which remdesivir and hydroxychloroquine will be compared.
Hydroxychloroquine was part of the World Health Organization's “Solidarity” study, which began in March 2020, and which also tests lopinavir / ritonavir (sometimes in combination with beta interferon ) and remdesivir . However, the World Health Organization announced on May 25, 2020 that the administration of hydroxychloroquine as part of the study would be suspended for the time being as long as safety concerns were not clarified. A study published in the journal "The Lancet" had come to the conclusion that the use of hydroxychloroquine and chloroquine could not be proven to be beneficial, but that severe side effects and an increased risk of death could be expected instead. However, this study was withdrawn because glaring deficiencies were demonstrated and the figures were not plausible. As a result, the WHO resumed its studies on the effectiveness of the drug.
They are also part of the “Discovery” large-scale study that began at the end of March 2020, in the context of which remdesivir and lopinavir / ritonavir are to be compared with the previous treatment in more than 3000 Covid-19 patients in eight European countries.
On April 21, the results of a retrospective, non-randomized study were published in which the data from 368 patients in the United States were evaluated. 97 had received hydroxychloroquine, 113 additionally received azithromycin , 158 neither. The risk of death in patients treated with hydroxychloroquine alone was greater than in the untreated group. Patients treated with hydroxychloroquine and azithromycin had at least a similar risk of death as the untreated group. Patients in all three groups had to be ventilated similarly often.
On April 24, 2020, the FDA issued a Drug Safety Communication warning of complications that may be associated with the use of chloroquine and hydroxychloroquine. There have been reports of serious heart rhythm problems in Covid 19 patients treated with these substances. Those affected were often treated in combination with azithromycin or other QT-prolonging drugs. When using hydroxychloroquine or chloroquine in the context of emergency programs or clinical studies, she recommended an initial evaluation and monitoring of the therapy with, for example, ECG , electrolytes, as well as kidney function and liver tests.
At the beginning of May, a study was published on a small group of patients who received the preparation with mild symptoms. Hydroxychloroquine showed no benefit here. The elimination of the viral load was significantly faster in the control group (47.8% vs. 90.9%).
On May 8, 2020, Carlucci et al. a case study that provided indications of an effective COVID-19 therapy with simultaneous administration of hydroxychloroquine and zinc. Zinc ions intervene in the replication machinery of RNA viruses . It has been suggested that this combination be further explored in the framework of the WHO Solidarity study.
US President Donald Trump said on May 19, 2020 that he had been taking hydroxychloroquine for a few weeks as a prophylaxis against Covid-19, and on May 24, 2020 he announced that he had meanwhile stopped taking it. The President of El Salvador , Nayib Bukele , stated in late May 2020 that he was taking hydroxychloroquine as a prophylaxis.
The Brazilian Ministry of Health published a recommendation for doctors to treat Covid-19 with hydroxychloroquine on May 22, 2020.
At the end of May 2020, the Indian Council for Medical Research (ICMR) stated that it was recommending hydroxychloroquine for prophylaxis. It causes “no harm” and “might be useful”.
In early June 2020, a study by Oxford University found that hydroxychloroquine had no benefit in treating COVID-19. In the study with about 4,500 patients, a third had been given the drug, while the rest were treated normally. After 28 days, 25.7% of the hydroxychloroquine group died, while 23.5% of the other group died. The difference is not statistically relevant, but it can be clearly stated that hydroxychloroquine does not work for the treatment of COVID-19. On June 15, 2020, the FDA revoked the March 28 approval to use hydroxychloroquine as an agent against COVID-19.
Peter Gottfried Kremsner , head of the study at the University of Tübingen on the drug, warned against a premature condemnation of hydroxychloroquine. He didn't expect it to be a miracle drug, but he could very well imagine that it would be suitable for treatment. Harvey Risch from the Yale School of Public Health recommended taking the drug in combination with zinc and doxycycline at the end of May 2020 in the early stages of the disease, so severe cases could be avoided.
Hydroxychloroquine is considered the drug of choice in various African countries. So it is used in Morocco , Nigeria , Senegal , Algeria and Chad . There the use is judged to be very promising. The Minister of Health of Morocco Khalid Ait Taleb said that the use of hydroxychloroquine in Morocco effectively prevented deaths like in Europe.
Brazilian President Jair Bolsonaro was treated with hydroxychloroquine in July 2020 after testing positive for Covid-19.
According to a study from Detroit from July 2020, the use of hydroxychloroquine could massively reduce mortality in Covid-19 patients. This result was confirmed by another study. Dr. Harvey Risch of Yale University believes that early treatment with the drug could have saved thousands of victims in the United States. The Association of American Doctors subsequently demanded that hydroxychloroquine had to be released again for the treatment of Covid-19 and made available so that the drug could be taken preventively by many citizens. Another study from Spain supported the thesis that hydroxychloroquine is effective in the early phase of Covid-19 disease.
Trade names
Plaquenil (CH), Quensyl (D)
Web links
Individual evidence
- ^ The Merck Index. An Encyclopaedia of Chemicals, Drugs and Biologicals . 14th edition, 2006.
- ↑ There is not yet a harmonized classification for this substance . A labeling of hydroxychloroquine in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), which was retrieved on January 14, 2020, is reproduced from a self-classification by the distributor .
- ↑ emedicine.com
- ↑ Jing Xue, Amanda Moyer, Bing Peng, Jinchang Wu, Bethany N. Hannafon: Chloroquine Is a Zinc Ionophore . In: PLoS ONE . tape 9 , no. 10 , October 1, 2014, p. e109180 , doi : 10.1371 / journal.pone.0109180 , PMID 25271834 , PMC 4182877 (free full text) - ( plos.org [accessed on March 21, 2020]).
- ↑ Aartjan JW te Velthuis, Sjoerd HE van den Worm, Amy C. Sims, Ralph S. Baric, Eric J. Snijder: Zn2 + Inhibits Coronavirus and Arterivirus RNA-Polymerase Activity In Vitro and Zinc Ionophores Block the Replication of These Viruses in Cell Culture . In: PLOS Pathogens . tape 6 , no. 11 , April 11, 2010, p. e1001176 , doi : 10.1371 / journal.ppat.1001176 , PMID 21079686 , PMC 2973827 (free full text) - ( plos.org [accessed on March 21, 2020]).
- ↑ ZJ Yang, CE Chee et al. a .: The role of autophagy in cancer: therapeutic implications. In: Molecular cancer therapeutics. Volume 10, number 9, September 2011, pp. 1533-1541, doi : 10.1158 / 1535-7163.MCT-11-0047 , PMID 21878654 , PMC 3170456 (free full text) (review).
- ↑ External identifiers of or database links to hydroxychloroquine sulfate: CAS number: 747-36-4, EC number: 212-019-3, ECHA InfoCard: 100.010.927 , PubChem : 12947 , ChemSpider : 12410 , DrugBank : DBSALT000096 , Wikidata : Q27270879 . Molecular formula C 18 H 26 ClN 3 O · H 2 SO 4 . Molecular weight 434.0 g · mol -1 .
- ↑ EDQM (Ed.): European Pharmacopoeia 9.0 . Monograph "Hydroxychloroquine sulfate". 2017, p. 2721 f .
- ↑ Chhonker YS, Sleightholm RL, Li J, Oupický D, Murry DJ: Simultaneous quantitation of hydroxychloroquine and its metabolites in mouse blood and tissues using LC-ESI-MS / MS: An application for pharmacokinetic studies. , J Chromatogr B Analyt Technol Biomed Life Sci. 2018 Jan 1; 1072: 320-327, PMID 29207305 .
- ↑ Füzéry AK, Breaud AR, Emezienna N, Schools S, Clarke WA: A rapid and reliable method for the quantitation of hydroxychloroquine in serum using turbulent flow liquid chromatography-tandem mass spectrometry. , Clin Chim Acta. 2013 Jun 5; 421: 79-84, PMID 23485647 .
- ↑ Yeon Lee J, Lee J, Ki Kwok S, Hyeon Ju J, Su Park K, Park SH: Factors Related to Blood Hydroxychloroquine Concentration in Patients With Systemic Lupus Erythematosus. , Arthritis Care Res (Hoboken). 2017 Apr; 69 (4): 536-542, PMID 27390146 .
- ↑ Kemmenoe AV: An infant fatality due to hydroxychloroquine poisoning. , J Anal Toxicol. 1990 May-Jun; 14 (3): 186-188, PMID 2374409 .
- ↑ Heimann, Heinrich et al .: Atlas of the fundus. Chapter 7: Macular Disorders , Section 7.7: Chloroquine and Hydroxychloroquine Retinopathy . Thime, 2010. doi : 10.1055 / b-0034-40509 .
- ↑ Richard Bergholz: Chloroquine Maculopathy: Risk Factors and Early Detection. Habilitation thesis. Medical Faculty Charité - Universitätsmedizin Berlin, November 2017. PDF .
- ↑ a b c Specialist information Quensyl®. Sanofi-Aventis Deutschland GmbH, April 2019, accessed on April 24, 2020 .
- ↑ a b G. Geisslinger et al .: Mutschler drug effects . 11th edition. WVG, Stuttgart 2019, p. 798 .
- ↑ Xueting Yao, Fei Ye, Miao Zhang, Cheng Cui, Baoying Huang, Peihua Niu, Xu Liu, Li Zhao, Erdan Dong, Chunli Song, Siyan Zhan, Roujian Lu, Haiyan Li, Wenjie Tan, Dongyang Liu: In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) . In: Clinical Infectious Diseases . March 9, 2020, doi : 10.1093 / cid / ciaa237 ( forthcoming , pre-release).
- ↑ Jia Liu, Ruiyuan Cao, Mingyue Xu, Xi Wang, Huanyu Zhang, Hengrui Hu, Yufeng Li, Zhihong Hu, Wu Zhong, Manli Wang: Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro . In: Cell Discover . tape 6 , March 18, 2020, doi : 10.1038 / s41421-020-0156-0 .
- ↑ Chen Jun, Liu Danping, Liu Li, Liu Ping, Xu Qingnian, Xia Lu, Ling Yun, Huang Dan, Song Shuli, Zhang Dandan, Qian Zhiping, Li Tao, Shen Yinzhong, Lu Hongzhou: A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19) . In: Journal of Zhejiang University (Medical Sciences) . tape 49 , no. 1 , March 3, 2020, doi : 10.3785 / j.issn.1008-9292.2020.03.03 ( com.cn [PDF]).
- ↑ Philippe Gautret, Jean-Christophe Lagier, Philippe Parola, Van Thuan Hoang, Line Meddeb, Morgane Mailhe, Barbara Doudier, Johan Courjon, Valérie Giordanengo, Vera Esteves Vieira, Hervé Tissot Dupont, Stéphane Honoré, Philippe Colson, Eric Chabrière, Bernard La Scola, Jean-Marc Rolain, Philippe Brouqui, Didier Raoult: Hydroxychloroquine and azithromycin as a treatment of COVID ‐ 19: results of an open ‐ label non ‐ randomized clinical trial . In: International Journal of Antimicrobial Agents . doi : 10.1016 / j.ijantimicag.2020.105949 .
- ↑ Hanno Böck: About that Hydroxychloroquine for COVID-19 trial. In: Better Science. March 23, 2020, accessed March 23, 2020 .
- ↑ Anja Martini: Malaria drug no hope for the time being. In: The coronavirus update with Christian Drosten. NDR Info, March 19, 2020, pp. 2–4 , accessed on March 20, 2020 (podcast transcript).
- ↑ Therapy review - CHLOROQUIN AND HYDROXYCHLOROQUIN - RAPIDLY UPGRADE IN COVID-19. In: arznei-telegram. April 24, 2020, accessed April 27, 2020 .
- ↑ Statement on IJAA paper - Official Statement from the International Society of Antimicrobial Chemotherapy (ISAC). International Society of Antimicrobial Chemotherapy, April 3, 2020, accessed April 27, 2020 .
- ^ "He was a science star. Then he promoted a questionable cure for Covid-19" In: New York Times, dated: May 12, 2020
- ↑ Zhaowei Chen, Jijia Hu, Zongwei Zhang, Shan Jiang, Shoumeng Han, Dandan Yan, Ruhong Zhuang, Ben Hu, Zhan Zhang: Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. (PDF) March 22, 2020, accessed April 4, 2020 .
- ↑ Anja Martini, Christian Drosten : (27) Coronavirus update: Mobile phone apps can offer a perspective. In: ndr.de. Norddeutscher Rundfunk, April 3, 2020, accessed on April 4, 2020 .
- ↑ a b Ulla Thiede: Do we have a drug against Covid-19 soon? In: general-anzeiger-bonn.de. April 10, 2020, accessed April 13, 2020 .
- ↑ Trump's tweet from April 21, 2020. In: twitter.com. March 21, 2020, accessed April 22, 2020 .
- ↑ Riley Beggin: Trump keeps promoting an unproven coronavirus treatment - despite his experts' advice. In: Vox.com. Vox Media, March 21, 2020, accessed March 23, 2020 .
- ↑ Werner Bartens: Coronavirus: Bad advice from the president. Retrieved April 6, 2020 .
- ↑ Lisette Voytko: New York To Begin Clinical Trials For Coronavirus Treatment Tuesday, Cuomo Says. In: Forbes. March 22, 2020, accessed March 23, 2020 .
- ↑ Erika Edwards, Vaughn Hillyard: Man dies after ingesting chloroquine in an attempt to prevent coronavirus. NBC News, March 23, 2020, accessed March 24, 2020 .
- ↑ Instituut voor Tropische Geneeskunde, University of Antwerp, UMC Sint-Pieter, Sciensano, Federaal Agentschap voor Geneesmiddelen en Gezondheidsproducten (Ed.): Interim Clinical Guidance for Patients Suspected of / Confirmed with COVID-19 in Belgium . Version 4. March 19, 2020 ( wiv-isp.be [PDF; accessed March 23, 2020]).
- ↑ Kwak Sung-sun: Physicians work out treatment guidelines for coronavirus. In: Korea Biomedical Review. February 13, 2020, accessed March 23, 2020 .
- ↑ a b Coronavirus SARS-CoV-2. In: bfarm.de. Federal Institute for Drugs and Medical Devices, 2020, accessed on April 10, 2020 (the page is updated regularly, see also versions from March 25 and April 10 in the Web Archive).
- ↑ Information on individual healing attempts with hydroxychloroquine in inpatients with COVID-19. (PDF) In: bfarm.de. Federal Institute for Drugs and Medical Devices , April 3, 2020, accessed on April 6, 2020 (the first publication date of March 25th can be found on the archived page ).
- ↑ COVID-19: chloroquine and hydroxychloroquine only to be used in clinical trials or emergency use programs. In: ema.europea.eu. European Medicines Agency , April 1, 2020, accessed April 3, 2020 .
- ↑ COVID-19: reminder of risk of serious side effects with chloroquine and hydroxychloroquine , News, EMA of April 23, 2020, accessed April 30, 2020.
- ↑ T. Dingermann : Supply bottlenecks for hydroxychloroquine and chloroquine feared , Pharmazeutische Zeitung, April 3, 2020.
- ↑ Hydroxychloroquine - Ensuring the care of chronically ill patients in the approved indications. (PDF) Federal Institute for Drugs and Medical Devices , April 3, 2020, accessed on April 6, 2020 .
- ↑ Hydroxychloroquine: Risk of serious side effects when used to treat COVID-19 , Other drug risks , BfArM dated April 29, 2020, accessed on April 30, 2020.
- ↑ Hydroxychloroquine - repeal of the regulations and recommendations to ensure the care of chronically ill patients in the approved indications. In: bfarm.de. Federal Institute for Drugs and Medical Devices , July 7, 2020, accessed on July 7, 2020 .
- ↑ “Solidarity” clinical trial for COVID-19 treatments. World Health Organization, accessed May 26, 2020 .
- ↑ Covid-19: WHO suspends study with hydroxychloroquine. In: Pharmaceutical newspaper. May 25, 2020, accessed May 26, 2020 .
- ↑ WHO halts trials of 'Trump drug' over safety fears . In: BBC News . May 25, 2020 (English, bbc.com [accessed May 26, 2020]).
- ^ "Specialist journal" The Lancet "withdraws study on malaria drugs" , in: Der Tagesspiegel, from June 5, 2020.
- ↑ "Three authors withdraw results from Corona study" , in: Der Spiegel, June 5, 2020.
- ↑ AFP: WHO resumes clinical studies with hydroxychloroquine. June 3, 2020, accessed June 5, 2020 .
- ↑ Julia Koch: Insane speed . In: Der Spiegel . No. 14 , 2020, p. 106 ( online - March 28, 2020 ).
- ↑ Joseph Magagnoli, Siddharth Narendran, Felipe Pereira, Tammy Cummings, James W. Hardin, S. Scott Sutton, Jayakrishna Ambati: Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. (PDF) In: medrxiv.org. April 21, 2020, accessed on April 23, 2020 .
- ↑ FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems , FDA, April 24, 2020.
- ↑ Jihad Mallat et al .: Hydroxychloroquine is associated with slower viral clearance in clinical COVID-19 patients with mild to moderate disease: A retrospective study , medRxiv (Preprint), 2020-05-02, doi: 10.1101 / 2020.04.27.20082180 .
- ↑ Philip Carlucci, Tania Ahuja, Christopher M. Petrilli, Harish Rajagopalan, Simon Jones: Hydroxychloroquine and azithromycin plus zinc vs hydroxychloroquine and azithromycin alone: outcomes in hospitalized COVID-19 patients . In: medRxiv . May 8, 2020, p. 2020.05.02.20080036 , doi : 10.1101 / 2020.05.02.20080036 ( medrxiv.org [accessed June 11, 2020]).
- ↑ R. the wall, M. Scholz: Does zinc supplementation enhance the clinical efficacy of chloroquine / hydroxychloroquine to win today's battle against COVID-19? In: Medical Hypotheses . tape 142 , May 6, 2020, ISSN 0306-9877 , p. 109815 , doi : 10.1016 / j.mehy.2020.109815 , PMID 32408070 , PMC 7202847 (free full text) - ( sciencedirect.com [accessed June 11, 2020]).
- ↑ "Gift of God" against Corona? Trump takes hydroxychloroquine as prophylaxis. In: n-tv.de. May 19, 2020, accessed May 19, 2020 .
- ↑ Coronavirus agent: WHO suspends clinical tests with hydroxychloroquine . In: FAZ.NET . May 26, 2020 ( faz.net [accessed May 26, 2020]).
- ^ "Salvadoran leader says he takes hydroxychloroquine," cnn.com, May 28, 2020.
- ↑ Brazil relies on controversial therapy. In: n-tv.de. May 22, 2020, accessed May 22, 2020 .
- ↑ "India uses controversial malaria drug against Corona" , in: Die Zeit, from May 27, 2020.
- ↑ Sarah Boseley: "Hydroxychloroquine does not cure Covid-19, say drug trial chiefs" The Guardian of June 5, 2020
- ^ Revocation of Approval , FDA, June 15, 2020
- ↑ Deutsche Welle June 5, 2020: Confusing debate about hydroxychloroquine
- ^ "Early Outpatient Treatment of Symptomatic, High-Risk Covid-19 Patients that Should be Ramped-Up Immediately as Key to the Pandemic Crisis" in: American Journal of Epidemiology, from: May 27, 2020
- ↑ "Ces pays africains qui ont décidé de continuer à soigner le Covid-19 avec l'hydroxychloroquine" in: Franceinfo, from: May 29, 2020
- ^ "President Bolsonaro infected with coronavirus" , in: Deutschlandfunk, July 8, 2020.
- ^ "Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19" in: International Journal of Infectious Diseases of July 1, 2020.
- ↑ "COVID-19 Outpatients - Early Risk-Stratified Treatment with Zinc Plus Low Dose Hydroxychloroquine and Azithromycin: A Retrospective Case Series Study", from July 3, 2020 in: Preprints 2020, 2020070025 (doi: 10.20944 / preprints202007.0025.v1)
- ^ "Hydroxychloroquine could save up to 100,000 lives if used for COVID-19: Yale epidemiology professor", July 22, 2020 in: Fox News
- ↑ "More Evidence Presented for Why Hydroxychloroquine Should be Made Available, in a New Court Filing by the Association of American Physicians & Surgeons" of July 22, 2020, in: Association of American Physicians and Surgeons (AAPS) - press release
- ↑ "Observational Study of the Efficiency of Treatments in Patients Hospitalized with Covid-19 in Madrid" in: medrxiv, https://doi.org/10.1101/2020.07.17.20155960 of July 21, 2020