Magnesium orthosilicate
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
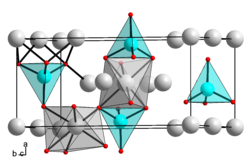
|
||||||||||||||||
| __ Mg 2+ __ Si 4+ __ O 2− | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Magnesium orthosilicate | |||||||||||||||
| other names |
Dimagnesium silicate |
|||||||||||||||
| Ratio formula | Mg 2 SiO 4 | |||||||||||||||
| Brief description |
white solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 140.69 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
3.22 g cm −3 |
|||||||||||||||
| Melting point |
1898 ° C |
|||||||||||||||
| solubility |
practically insoluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Magnesium orthosilicate is a chemical compound of magnesium from the group of silicates .
Occurrence
Magnesium orthosilicate occurs naturally in the form of the mineral forsterite , ringwoodite, and wadsleyite . The compound forms with the iron silicate fayalite (Fe 2 SiO 4 ) a complete series of mixtures within the Oliving group . In this form it is a main component of the earth's mantle .
Extraction and presentation
Magnesium orthosilicate can be obtained by reacting magnesium with silicon dioxide nanoparticles at 1100 ° C in argon . It is also possible to display the reaction by reaction of tetramethoxysilane or tetraethoxysilane with water and magnesium in methanol or the sol-gel process of magnesium nitrate with colloidal silicon dioxide.
properties
Magnesium orthosilicate is a white solid that is practically insoluble in water. The most common form of the compound (forsterite) has an orthorhombic crystal structure of the olivine type space group Pnma (space group no.62 ) , lattice parameters a = 0.4756 nm, b = 1.0197 nm, c = 0.5982 nm. Under high pressure ( Temperatures above 1000 ° C and pressure above 12 GPa) can also result in two other modifications that are also stable under normal conditions. These correspond to the minerals wadsleyite with an orthorhombic crystal structure and the space group Imma (space group no. 74) and ringwoodite with a cubic crystal structure and the space group Fd 3 m (space group no. 227) . From 33 GPa and 1000 ° C, the compound decomposes into magnesium oxide and silicon dioxide.
Individual evidence
- ↑ a b c d e Dale L. Perry: Handbook of Inorganic Compounds, Second Edition . CRC Press, 2016, ISBN 978-1-4398-1462-8 , pp. 257 ( limited preview in Google Book search).
- ^ A b William M. Haynes: CRC Handbook of Chemistry and Physics, 94th Edition . CRC Press, 2016, ISBN 978-1-4665-7115-0 , pp. 73 ( limited preview in Google Book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Anna Hansen: Structure and stability of silicates . GRIN Verlag, 2010, ISBN 978-3-640-57164-2 , p. 4 ( limited preview in Google Book search).
- ↑ Harry G. Brittain: Profiles of Drug Substances, Excipients and Related Methodology . Academic Press, 2011, ISBN 978-0-12-387667-6 , pp. 281 ( limited preview in Google Book search).
- ↑ E. Yu Tonkov: High Pressure Phase Transformations Handbook 1 . CRC Press, 1992, ISBN 978-2-88124-758-3 , pp. 543 ( limited preview in Google Book search).
- ↑ Surendra K. Saxena, Nilanjan Chatterjee, Yingwei Fei, Guoyin Shen: Thermodynamic Data on Oxides and Silicates An Assessed Data Set Based on Thermochemistry and High Pressure Phase Equilibrium . Springer Science & Business Media, 2012, ISBN 978-3-642-78332-6 , p. 58 ( limited preview in Google Book search).