Phthalic anhydride
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
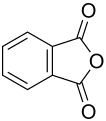
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Phthalic anhydride | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 8 H 4 O 3 | ||||||||||||||||||
| Brief description |
colorless to whitish crystal needles with an aromatic odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 148.12 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.53 g cm −3 (20 ° C) |
||||||||||||||||||
| Melting point |
131 ° C |
||||||||||||||||||
| boiling point |
285 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
Switzerland: 1 mg m −3 |
||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Phthalic anhydride (according to IUPAC nomenclature : 2-benzofuran-1,3-dione , also called PSA for short ) is an organic-chemical compound from the group of aromatic carboxylic acid anhydrides , more precisely it is the anhydride of phthalic acid . The compound is an important starting material for the production of synthetic resins , as well as dyes and color pigments . It is also one of the basic chemicals and is used as an intermediate in the chemical industry .
Extraction and presentation
Industrial synthesis
The large-scale production of phthalic anhydride takes place today through the catalytic oxidation of o -xylene with atmospheric oxygen in the gas phase at temperatures of 375-410 ° C. The catalysts used are mixtures of vanadium pentoxide (V 2 O 5 ) and titanium dioxide (TiO 2 ), which are supported on ceramic rings and in some cases can contain promoters .
The complete reaction is carried out in tube bundle reactors , in which the considerable heat of reaction (ΔH R = –1110 kJ · mol −1 ) is dissipated with the aid of molten salts and used to generate superheated high-pressure steam. In this process, the catalyst is arranged as a fixed bed . The yield of phthalic anhydride is 87% and it is obtained after two-stage distillation with a purity of 99.8%.
Until the 1960s, phthalic anhydride was produced using the Gibbs-Wohl process by air oxidation of naphthalene , which in turn was obtained from coal tar .
The production capacities for phthalic anhydride in 2010 were around 3.0 million tonnes per year worldwide .
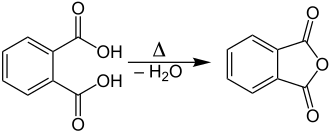
Laboratory scale
In the laboratory, phthalic anhydride can be produced by heating phthalic acid (C 8 H 6 O 4 ) with elimination of water :
Under suitable conditions - preferably reduced pressure - the phthalic anhydride distills or sublimates and condenses without the water in a receiver.
properties
Physical Properties
Phthalic anhydride has a relative gas density of 5.11 (density ratio to dry air at the same temperature and pressure ). In addition, it does not dissolve very much in cold water , ethanol and diethyl ether , but it dissolves well in esters , ketones , halogenated hydrocarbons and benzene . Technical phthalic anhydride or those stored in containers that are not completely tightly sealed can contain a considerable proportion of phthalic acid , as the anhydride is slowly converted to acid with the humidity in the air. Then it has to be distilled or sublimed, preferably at reduced pressure.
Chemical properties
Phthalic anhydride is a combustible, but hardly ignitable solid from the group of carboxylic acid anhydrides . In contact with oxidizing agents , nitric acid , glycerine (heat), copper oxide (heat), air (phthalic anhydride dust), sodium nitrite (heat), explosive reactions can occur. Dangerous chemical reactions can occur with strong bases , alcohols in connection with heat, metals and hot water .
use
Phthalic anhydride is a large-scale industrial mass product that has numerous applications. It is mainly used as a raw material for the production of plasticizers in the form of phthalic acid esters for plastics (especially PVC ). It is also an important intermediate in the production of esters , alkyd resins , polyester resins , polyimides , phthalocyanine dyes and heterocycles . It also serves as a fine chemical and precursor for functionalized aromatics , anthraquinones , anthraquinone dyes , indanthrene dyes , xanthene dyes , phenolphthalein and phthalimides . Isatoic anhydride and saccharine are also produced from phthalic anhydride . It is also used as a crosslinker for epoxy resins , in printing inks, paints , photographic materials, lithographic printing plates, fuel additives and coating materials. It is also used in small quantities to modify polymers , wood and cellulose .
safety instructions
The dust of phthalic anhydride can form explosive mixtures with air if finely divided and in the presence of an ignition source. Phthalic anhydride is mainly absorbed through the respiratory tract . In addition, a possibility of absorption via the digestive tract has been proven. Ingestion or exposure causes acute irritation to corrosive effects on the eyes , respiratory tract and skin . In addition, there is a sensitizing effect (preferably inhalation) and immediate allergic reactions. Chronic it can lead to allergic respiratory diseases such as rhinitis or bronchial asthma . A mutagenicity could be excluded in microbiological tests . There is currently insufficient information on reproductive toxicity and carcinogenicity . Phthalic anhydride has a lower explosion limit (LEL) of 1.7 vol.% (100 g / m 3 ) and an upper explosion limit (UEL) of 10.5 vol.% (650 g / m 3 ). The lower explosion point is 136 ° C. The ignition temperature is 580 ° C. With a flash point of 152 ° C, phthalic anhydride is considered difficult to ignite.
Individual evidence
- ↑ a b c d e f g h i j k Entry on phthalic anhydride in the GESTIS substance database of the IFA , accessed on July 22, 2019(JavaScript required) .
- ↑ a b c d e f Entry on phthalic anhydride. In: Römpp Online . Georg Thieme Verlag, accessed on July 22, 2019.
- ↑ Entry on Phthalic anhydride in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on July 22, 2019. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Schweizerische Unfallversicherungsanstalt (Suva): Limits - Current MAK and BAT values (search for 85-44-9 or phthalic anhydride ), accessed on October 5, 2019.
- ↑ a b Manfred Baerns, Arno Behr, Axel Brehm, Jürgen Gmehling, Kai-Olaf Hinrichsen, Hanns Hofmann, Regina Palkovits, Ulfert Onken, Albert Renken: Technische Chemie . 2nd Edition. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany 2013, ISBN 978-3-527-33072-0 , p. 605 .
- ^ Siegfried Hauptmann : Organic Chemistry , 2nd reviewed edition, VEB Deutscher Verlag für Grundstoffindindustrie, Leipzig, 1985, p. 441, ISBN 3-342-00280-8 .