Plaque assay
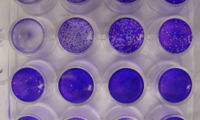
A plaque assay (French. Plaque [plak] for "plate; stain; sign") is a method for the detection and quantitation of infectious cytopathic virus in which a to be examined sample at various dilutions in a cell culture is introduced.
principle
Because of a cytopathic effect occurs after infection of cells with virus particles (synonymously virions ) for lysis of the cells and their immediate neighboring cells. After these cells have been lysed, the virus particles that have been released reach neighboring cells, causing the virus to spread further and further. This leads to a spot in a confluent cell lawn in which the cells were lysed. The holes in the cell lawn are called Lysishof or plaque . If an infected cell lawn is fixed after a reasonable time in which such plaques could form and then stained and washed with methylene blue , crystal violet or neutral red , the plaques can be recognized as empty, unstained areas from which the dead, lysed cells emerge have partially peeled off during washing. The plaques are counted and together with the known volume used and the dilution factor , the concentration of infectious virus particles in the sample is determined, typically as infectious units per milliliter, IU / ml, or plaque forming units per milliliter, PFU / ml.
In contrast to the determination of the concentration of viruses in the blood or serum in terms of a viral load by means of detection of the viral DNA or RNA by PCR and possibly a preceding reverse transcription , in the plaque assay inactivated or non-infectious particles within a virus population are not recorded. The plaque assay is therefore a direct measure of the concentration of infectious particles in a sample. However, since not all infectious virions placed on the cells also lead to an infection (after the virus has been added and before the polymer has been added), and not all virus particles reach the cell lawn far enough apart, the plaque assay tends to determine values that are too low.
To improve the accuracy of the method, the cells can be covered with a polymer (e.g. low-melting agarose or cellulose powder ) after infection . This coating prevents unintentional infection of cells via the culture medium during the three days of the experiment in the event of shocks or vibrations and only allows direct infections of neighboring cells, as otherwise plaques of daughter viruses of the following generations could possibly develop via the medium and an excessively high concentration would be determined . Since low-melting agarose has to be heated to around 50 ° C, there is no risk of the cells being heated in comparison with cellulose powder.
In the case of viruses that have no cytopathic effect, the virus concentration can be determined via the tissue culture infectious dose of 50% (TCID 50 , synonym cell culture infectious dose , CCID 50 ). This method is based on the infection of the cell culture and any kind of detection (e.g. indirect immunoperoxidase assay , immunofluorescence test , DNA extraction with PCR , RNA extraction with RT-PCR ) whether a cell culture has occurred after a long period of time (e.g. seven days) is infected at all. The determination of the TCID 50 avoids the problem of infection by daughter viruses by avoiding a count of plaques, since only the limit dilutions at which an infection still takes place are determined.
Applications
With the plaque assay only virus particle concentrations of viruses can be detected which can infect a cell line used, replicate in it and also lead to the lysis of the cells such as e.g. B. Influenza viruses in MDCK cells . In the case of lytic viruses , the plaque assay can also be used to determine the viral load , a virus titer or the minimum infection dose.
In addition to determining the concentration of infectious virions, the plaque assay can also be used in a virus neutralization assay (synonymous plaque reduction assay) to detect infection-inhibiting antibodies against parts of the virion . For this purpose, viruses are incubated in a known concentration with a sample to be tested for neutralizing antibodies and then applied to the cells. If the antibodies bind to those surface epitopes of the viruses that are necessary for uptake into the cell, a reduction in the PFU / mL can be seen in the plaque assay compared to a negative control without these antibodies.
The plaque assay is also used to investigate the effect of disinfectants in which the viral genome is not impaired and remains detectable unchanged, but the infectivity of the virions is reduced. The plaque assay can also be used to examine how high the concentration of infectious viruses is in a collected sample or after virus cultivation in the cell culture medium. With many viruses (especially RNA viruses ), only a small percentage of the virions released are actually infectious.
The forerunner of the plaque assay recorded the number of focus forming units per milliliter in the case of influenza viruses based on the change in the amnion of infected embryonated chicken eggs .
literature
- HL Bachrach, JJ Callis et al .: A plaque assay for foot-and-mouth disease virus and kinetics of virus reproduction . Virology (1957) 4 (2): pp. 224-36 PMID 13496542
- PD Cooper: The plaque assay of animal viruses . Adv Virus Res (1961) 8: pp. 319-78 PMID 13881155
Individual evidence
- ↑ MB Gonzalez-Hernandez, J. Bragazzi Cunha, CE Wobus: Plaque assay for murine norovirus. In: Journal of visualized experiments: JoVE. Number 66, 2012, p. E4297, ISSN 1940-087X . doi : 10.3791 / 4297 . PMID 22951568 . PMC 3487293 (free full text).
- ↑ CR Gaush, TF Smith: Replication and plaque assay of influenza virus in at established line of canine kidney cells. Appl Microbiol. 16 (4): 588-94 (1968). PMID 5647517 .
- ↑ M. Matrosovich, T. Matrosovich, W. Garten, HD Klenk: New low-viscosity overlay medium for viral plaque assays. Virol J. 3:63 (2006). PMID 16945126 .
- ↑ Y. Gao, I. Nankya, A. Abraha, RM Troyer, KN Nelson, A. Rubio, EJ Arts: Calculating HIV-1 Infectious Titre Using a Virtual TCID50 method. Methods in Molecular Biology Vol. 485 Page 27-35 (2008). PMID 19020816 .
- ^ AJ Eisfeld, G. Neumann, Y. Kawaoka: Influenza A virus isolation, culture and identification. In: Nature protocols. Volume 9, number 11, November 2014, pp. 2663–2681, doi : 10.1038 / nprot.2014.180 , PMID 25321410 .
- ↑ M. de Graaf, S. Herfst, EJ Schrauwen et al .: An improved plaque reduction virus neutralization assay for human metapneumovirus. J Virol Methods 143 (2): pp. 169-74 (2007). PMID 17420056 .
