Polybutylene succinate
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|
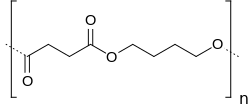
|
|||||||
| General | |||||||
| Surname | Polybutylene succinate | ||||||
| other names |
PBS |
||||||
| CAS number | 25777-14-4 | ||||||
| Monomers | 1,4-butanediol and succinic acid | ||||||
| Molecular formula of the repeating unit | C 8 H 12 O 4 | ||||||
| Molar mass of the repeating unit | 172 g mol −1 | ||||||
| Type of polymer |
Thermoplastic |
||||||
| properties | |||||||
| Physical state |
firmly |
||||||
| density |
1.24-1.28 g / cm 3 |
||||||
| Glass temperature |
−45 to −32 ° C |
||||||
| modulus of elasticity |
300-950 MPa |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Polybutylene succinate (PBS) is a chemical compound from the group of linear aliphatic polyesters .
Extraction and presentation
Polybutylene succinate can be obtained by reacting succinic acid with 1,4-butanediol. The starting materials (succinic acid and 1,4-butanediol) can be produced from both fossil and glucose .
properties
Polybutylene succinate is a linear aliphatic polyester. It is biodegradable - even in fresh and sea water - and forms water and CO 2 in the process . The biodegradability is better than that of PLA . PBS is not soluble in water, but it is soluble in chloroform.
Depending on the type, the properties of the material are roughly comparable to those of LDPE or polypropylene . PBS has a high long-term service temperature range from −40 to approx. 115 ° C, high impact strength and can be used in the food sector. It is also easy to weld and print well with both water-soluble and solvent-based inks. PBS can be processed into multilayer films by thermoforming either alone or together with other biodegradable plastics.
use
Due to its biodegradability, polybutylene succinate is used, for example, for packaging, cutlery, mulch films or medical articles.
It is also used as a matrix for biogenic composite materials. It is used as a material for automobile interior components, for example. One advantage is that such materials have much lower VOC emissions than conventional ones.
Individual evidence
- ↑ a b c d e f g Peter Schwarzmann: Thermoforming in practice . Carl Hanser Verlag GmbH Co KG, 2016, ISBN 978-3-446-44948-0 , p. 115 ( limited preview in Google Book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b c Oliver Türk: Material use of renewable raw materials Basics - Materials - Applications . Springer-Verlag, 2013, ISBN 978-3-8348-2199-7 , pp. 551 ( limited preview in Google Book search).
