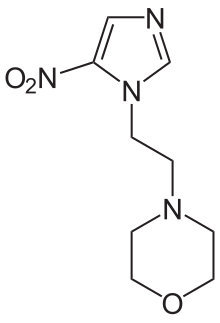
Radio sensitizer
A radiosensitizer (the term radiation sensitizer is hardly established in the German-language specialist literature ) is a drug that selectively increases the sensitivity of malignant tumor cells to ionizing radiation after administration . Radio sensitizers are a subgroup of radio modulators . Radioprotectors, on the other hand, are substances that selectively protect normal healthy tissue and thus enable a higher radiation dose.
description
One of the biggest problems with conventional radiation therapy is the low sensitivity of many tumors to radiation compared to the surrounding healthy tissue. The main reason for the low sensitivity to radiation is the lack of oxygen ( hypoxia ) in many tumors, which is caused by the rather moderate formation of new blood vessels ( angiogenesis ) during tumor growth. Oxygen is a very effective radio sensitizer. In vitro , oxygenated cells are 2 to 3 times more sensitive to ionizing radiation than the same cells in a hypoxic environment. The ratio of the radiation dose without a radiosensitizer to the radiation dose with a radiosensitizer, with the same in-vitro or in-vivo effect, is referred to as the sensitizer enhancement ratio (SER). The SER is a measure of the change in the effect of radiation by a radio sensitizer. In order to increase the sensitivity of tumors to radiation, various concepts and a number of different substances (radiosensitizers) have been developed. Many substances and processes are still in clinical testing .
"Cytostatics with a synergistic effect on radiation therapy", such as cisplatin , 5-fluorouracil , vindesine , hydroxyurea , doxorubicin or actinomycin D , have to be distinguished from the "real" radiosensitizers .
Therapy approaches and substance classes
There is a clear discrepancy between the results obtained in clinical studies to date and the preclinical data ( in vitro and in vivo ) . As a result, there is as yet no recommendation for a radio sensitizer that is supported by a broad consensus in the professional world.
Imitation of the oxygen effect
One therapeutic approach to improve radiosensitization is to imitate the oxygen effect in the tumor. Attempts are made to increase the oxygen content in cancer cells in order to increase their sensitivity to ionizing radiation.
One procedure is hyperbaric oxygenation directly before radiation. Hydrogen peroxide has similar effects .
Substances with an affinity for electrons, such as misonidazole , nimorazole , sanazole , azomycin (2-nitroimidazole), metronidazole or pimonidazole from the group of nitroimidazoles , are supposed to imitate the oxygen effect in the malignant tissue. SER values of 1.3 to 2.7 were found in model organisms . In Denmark, nimorazole is used against cancer of the throat and larynx in radiation therapy, because after capturing a slow electron, the molecule binds as a radical to DNA that has been damaged by radiation, thus disrupting DNA repair in tumor cells. In cells where there is enough oxygen, nimorazole releases the captured electron back into the oxygen and is recycled. This means that the radical accumulates in oxygen-poor cells.
As a radiosensitizer, efaproxiral (RFR13) changes the absorption of oxygen in hemoglobin , which therefore binds less to hemoglobin and is more easily released to hypoxic tissue. Efaproxiral is an allosteric effector of hemoglobin. The SER values are 1.8 to 2.1. Efaproxiral increases the cytotoxic effect of radiation therapy and chemotherapy with cytostatics . In the summer of 2007, development of efaproxiral, which was in clinical phase III, was discontinued. There was no significant survival benefit in breast cancer patients compared to women who received radiation therapy only.
DNA sensitizer
Another approach is followed with DNA sensitizers. With substances such as bromodeoxyuridine - a thymidine - analogue - is in the cell nucleus , the number of DNA single strand breaks increased. It is also possible that the intracellular repair mechanisms are disrupted. However, the impairment of healthy cells with a high rate of proliferation by bromodeoxyuridine is unfavorable. Even gold - nanoparticles seem to inhibit the location in the cell nucleus, the DNA repair mechanisms.
Cytarabine , or its metabolic product cytosine arabinoside triphosphate , acts as a radio sensitizer by inhibiting the repair mechanisms of cancer cells. This happens via the inhibition of the DNA polymerases .
Multifunctional radio sensitizer
Dexrazoxane (ICRF-187), an EDTA derivative, blocks the cell cycle in the early G 2 / M phase . In this phase, a cell has its highest radiation sensitivity. A normalization of the tumorous blood vessel system could also be determined in model organisms , which obviously improves the oxygen supply to the tumor. It also shows an anti- metastatic effect. It also significantly reduces the cardiotoxicity of anthracyclines . Dexrazoxane is approved for this application . In some studies, dexrazoxane was able to improve the prognosis of certain tumors in combination with radiation therapy . In contrast, the administration of dexrazoxane in cervical , bronchial and head and neck carcinomas had no advantages.
Also N methylformamide is a multifunctional radiosensitizer.
Thiol modulators
Thiol modulators are compounds that block thiol groups (-SH). These include N-ethyl maleimide , p- chloro mercuribenzoate and buthionine sulfoximine .
Further substance classes
In addition to these compounds, there is a large number of other classes of substances in (pre) clinical testing as radio sensitizers. These include resveratrol , hydroxychalcones and motexafin gadolinium (Gd-Tex). Motexafin gadolinium accumulates in the tumor after systemic administration and catalyzes the oxidation of reducing compounds such as ascorbic acid and glutathione in the cell . This triggers a reaction cascade, at the end of which reactive oxygen species are generated.
Medical history
As early as 1921, the German radiologist Hermann Holthusen made the observation that the radiation sensitivity of cells is increased by oxygen.
further reading
- Harrison LB, Chadha M., Hill RJ, Hu K., Shasha D.: Impact of tumor hypoxia and anemia on radiation therapy outcomes. In: Oncologist . Volume 7, Number 6, 2002, pp. 492-508, ISSN 1083-7159 , PMID 12490737 , (Review).
- R. Wideröe: Optimization of cancer radiotherapy with selective sensitizers. In: Radiation Therapy and Oncology . Volume 163, Number 6, June 1987, pp. 378-384, ISSN 0179-7158 , PMID 3603365 .
- S. Dische: Sensitizers in radiotherapy. In: British journal of hospital medicine. Volume 27, Number 5, May 1982, pp. 502, 507-502, 510, ISSN 0007-1064 , PMID 7093576 .
Individual evidence
- ↑ a b c J. Voges: Radiotherapy. In: U. Schlegel, M. Weller, M. Westphal (eds.): Neuroonkologie Verlag Thieme, ISBN 3-131-09062-6 , pp. 437-438 ( limited preview in Google book search).
- ↑ B. Kaser-Hotz: Principles of radiation therapy. In: M. Kessler (Ed.): Small animal oncology: diagnosis and therapy of tumor diseases in dogs. Georg Thieme Verlag, 2005, ISBN 3-830-44103-7 , p. 160 ( limited preview in Google book search).
- ^ DJ Lee, M. Moini, J. Giuliano, WH Westra: Hypoxic sensitizer and cytotoxin for head and neck cancer. In: Annals of the Academy of Medicine, Singapore. Volume 25, Number 3, May 1996, pp. 397-404, ISSN 0304-4602 , PMID 8876907 (review).
- ↑ LB Harrison, M. Chadha, RJ Hill, K. Hu, D. Shasha: Impact of tumor hypoxia and anemia on radiation therapy outcomes. In: The oncologist. Volume 7, Number 6, 2002, pp. 492-508, ISSN 1083-7159 , PMID 12490737 (review).
- ↑ a b c d e f g h i j W. Rhomberg, J. Dunst: Radiosensitizer. In: HJ Schmoll, K. Höffken, K. Possinger (Eds.): Compendium of internal oncology standards in diagnostics and therapy. Springer, 2005, ISBN 3-540-20657-4 , p. 619 ( limited preview in Google book search).
- ↑ K. Ogawa, S. Ishiuchi, O. Inoue, Y. Yoshii, A. Saito, T. Watanabe, S. Iraha, T. Toita, Y. Kakinohana, T. Ariga, G. Kasuya, S. Murayama: Phase II Trial of Radiotherapy after Hyperbaric Oxygenation with Multiagent Chemotherapy (Procarbazine, Nimustine, and Vincristine) for High-Grade Gliomas: Long-Term Results. In: International Journal of Radiation Oncology - Biology - Physics [electronic publication before print] March 2011, ISSN 1879-355X , doi : 10.1016 / j.ijrobp.2010.12.070 , PMID 21420247 .
- ↑ JP Kapp, A. Routh, D. Cotton: Hyperbaric oxygen as a radiation sensitizer in the treatment of brain tumors. In: Surgical Neurology . Volume 17, Number 3, March 1982, pp. 233-235, ISSN 0090-3019 , PMID 7079944 .
- ↑ Y. Ogawa, K. Kubota, H. Ue, Y. Kataoka, M. Tadokoro, K. Miyatake, K. Tsuzuki, T. Yamanishi, S. Itoh, J. Hitomi, N. Hamada, S. Kariya, M . Fukumoto, A. Nishioka, T. Inomata: Phase I study of a new radiosensitizer containing hydrogen peroxide and sodium hyaluronate for topical tumor injection: a new enzyme-targeting radiosensitization treatment, Kochi Oxydol-Radiation Therapy for Unresectable Carcinomas, Type II (KORTUC II). In: International Journal of Oncology . Volume 34, Number 3, March 2009, pp. 609-618, ISSN 1019-6439 , PMID 19212665 .
- ↑ M. Bamberg, P. Tamulevicius, C. Streffer, E. Scherer: Clinical experiences with the radiosensitizer misonidazole. In: Radiation Therapy. Volume 157, Number 8, August 1981, pp. 524-536, ISSN 0039-2073 , PMID 7268822 .
- ^ RP Hill: Sensitizers and radiation dose fractionation: results and interpretations. In: International journal of radiation oncology, biology, physics. Volume 12, Number 7, July 1986, pp. 1049-1054, ISSN 0360-3016 , PMID 2943705 , (Review).
- ↑ M. Bache, M. Kappler, HM Said, A. Staab, D. Vordermark: Detection and specific targeting of hypoxic regions within solid tumors: current preclinical and clinical strategies. In: Current Medicinal Chemistry . Volume 15, Number 4, 2008, pp. 322-338, ISSN 0929-8673 , PMID 18288988 , (Review).
- ↑ W. Dobrowsky, NG Huigol, RS Jayatilake, NI Kizilbash, S. Okkan, TV Kagiya, H. Tatsuzaki: AK-2123 (Sanazol) as a radiation sensitizer in the treatment of stage III cancer cervix: initial results of an IAEA multicentre randomized trial. In: Journal of Cancer Research and Therapeutics . Volume 1, Number 2, 2005 Apr-Jun, pp. 75-78, ISSN 1998-4138 , PMID 17998631 .
- ↑ R. Rajagopalan, TV Kagiya, CK Nair: radiosensitizer sanazole (AK-2123) Enhances gamma-radiation-induced apoptosis in murine fibrosarcoma. In: Journal of radiation research. Volume 44, Number 4, December 2003, pp. 359-365, ISSN 0449-3060 , PMID 15031563 .
- ↑ C. Sugie, Y. Shibamoto, M. Ito, H. Ogino, H. Suzuki, Y. Uto, H. Nagasawa, H. Hori: Reevaluation of the radiosensitizing effects of sanazole and nimorazole in vitro and in vivo. In: Journal of radiation research. Volume 46, Number 4, December 2005, pp. 453-459, ISSN 0449-3060 , PMID 16394636 .
- ↑ W. Dobrowsky, NG Huigol, RS Jayatilake, NI Kizilbash, S. Okkan, VT Kagiya, H. Tatsuzaki: AK-2123 (Sanazol) as a radiation sensitizer in the treatment of stage III cervical cancer: results of an IAEA multicentre randomized trial. In: Radiotherapy and Oncology . Volume 82, Number 1, January 2007, pp. 24-29, ISSN 0167-8140 . doi : 10.1016 / j.radonc.2006.11.007 , PMID 17161478 .
- ^ ME Watts, MF Dennis, IJ Roberts: Radiosensitization by misonidazole, pimonidazole and azomycin and intracellular uptake in human tumor cell lines. In: International journal of radiation biology. Volume 57, Number 2, February 1990, pp. 361-372, ISSN 0955-3002 , PMID 1968500 .
- ^ D. Nori, JM Cain, BS Hilaris, WB Jones, JL Lewis: Metronidazole as a radiosensitizer and high-dose radiation in advanced vulvovaginal malignancies, a pilot study. In: Gynecologic Oncology . Volume 16, Number 1, August 1983, pp. 117-128, ISSN 0090-8258 , PMID 6884824 .
- ^ DJ Chaplin, MR Horsman: Tumor blood flow changes induced by chemical modifiers of radiation response. In: International journal of radiation oncology, biology, physics. Volume 22, Number 3, 1992, pp. 459-462, ISSN 0360-3016 , PMID 1735678 .
- ^ DJ Chaplin: The effect of therapy on tumor vascular function. In: International journal of radiation biology. Volume 60, Numbers 1-2, 1991, pp. 311-325, ISSN 0955-3002 , PMID 1677988 , (Review).
- ↑ Rebecca Meißner, Jaroslav Kočišek, Eugen Illenberger, Stephan Denifl: Low-energy electrons transform the nimorazole molecule into a radiosensitiser. Nature Communications 10, 2388 (2019) doi : 10.1038 / s41467-019-10340-8
- ↑ BA Teicher, JS Wong, H. Takeuchi, LM Gravelin, G. Ara, D. Buxton: Allosteric effectors of hemoglobin as modulators of chemotherapy and radiation therapy in vitro and in vivo. In: Cancer Chemotherapy and Pharmacology . Volume 42, Number 1, 1998, pp. 24-30, ISSN 0344-5704 , PMID 9619754 .
- ^ ET Donnelly, Y. Liu, S. Rockwell: Efaproxiral (RSR13) plus oxygen breathing increases the therapeutic ratio of carboplatin in EMT6 mouse mammary tumors. In: Experimental biology and medicine (Maywood, NJ). Volume 231, Number 3, March 2006, pp. 317-321, ISSN 1535-3702 , PMID 16514179 .
- ^ RH Engel, VG Kaklamani: Role of efaproxiral in metastatic brain tumors. In: Expert Review of Anticancer Therapy . Volume 6, Number 4, April 2006, pp. 477-485, ISSN 1744-8328 . doi : 10.1586 / 14737140.6.4.477 , PMID 16613536 , (Review).
- ↑ C. Scott, J. Suh, B. Stea, A. Nabid, J. Hackman: Improved survival, quality of life, and quality-adjusted survival in breast cancer patients treated with efaproxiral (Efaproxyn) plus whole-brain radiation therapy for brain metastases. In: American Journal of Clinical Oncology . Volume 30, Number 6, December 2007, pp. 580-587, ISSN 1537-453X . doi : 10.1097 / COC.0b013e3180653c0d , PMID 18091051 .
- ↑ JH Suh, B. Stea, A. Nabid, JJ Kresl, A. Fortin, JP Mercier, N. Senzer, EL Chang, AP Boyd, PJ Cagnoni, E. Shaw: Phase III study of efaproxiral as an adjunct to whole- brain radiation therapy for brain metastases. In: Journal of clinical oncology : official journal of the American Society of Clinical Oncology. Volume 24, Number 1, January 2006, pp. 106-114, ISSN 1527-7755 . doi : 10.1200 / JCO.2004.00.1768 , PMID 16314619 .
- ↑ DB Chithrani, S. Jelveh, F. Jalali, M. van Prooijen, C. Allen, RG Bristow, RP Hill, DA Jaffray: Gold nanoparticles as radiation sensitizers in cancer therapy. In: Radiation research. Volume 173, Number 6, June 2010, pp. 719-728, ISSN 1938-5404 . doi : 10.1667 / RR1984.1 , PMID 20518651 .
- ^ E. Richter, T. Feyerabend: Basics of radiation therapy. Verlag Springer, 2002, ISBN 3-540-41265-4 , p. 80 ( limited preview in the Google book search).
- ^ GJ D'Angio: Regarding the alleged radiosensitization of intrathecal cytarabine. In: Journal of pediatric hematology / oncology. Volume 27, Number 7, July 2005, pp. 349-350, ISSN 1077-4114 , PMID 16012322 .
- ↑ K. Hellmann, W. Rhomberg: Radiotherapeutic enhancement by razoxane. In: Cancer Treatment Reviews . Volume 18, Number 4, December 1991, pp. 225-240, ISSN 0305-7372 , PMID 1842574 , (Review).
- ^ K. Hellmann, M. Goold, N. Higgins, RH Phillips: Responses of liver metastases to radiotherapy and razoxane. In: Journal of the Royal Society of Medicine. Volume 85, Number 3, March 1992, pp. 136-138, ISSN 0141-0768 , PMID 1556714 . PMC 1294812 (free full text).
- ↑ Dexrazoxane prevents the cardiotoxicity of anthracyclines. In: Ärzte Zeitung of July 20, 2007
- ↑ RC Kane, WD McGuinn, R. Dagher, R. Justice, R. Pazdur: Dexrazoxane (Totect): FDA review and approval for the treatment of accidental extravasation following intravenous anthracycline chemotherapy. In: The oncologist. Volume 13, Number 4, April 2008, pp. 445-450, ISSN 1083-7159 . doi : 10.1634 / theoncologist.2007-0247 , PMID 18448560 .
- ^ W. Rhomberg, H. Stephan, F. Böhler, K. Erhart, H. Eiter: Radiotherapy and razoxane in advanced bile duct carcinomas. In: Anticancer Research . Volume 25, Number 5, 2005 Sep-Oct, pp. 3613-3618, ISSN 0250-7005 , PMID 16101189 .
- ↑ W. Rhomberg, H. Eiter, F. Böhler, S. Dertinger: Combined radiotherapy and razoxane in the treatment of chondrosarcomas and chordomas. In: Anticancer Research . Volume 26, Number 3B, 2006 May-Jun, pp. 2407-2411, ISSN 0250-7005 , PMID 16821624 .
- ↑ K. Clagett-Carr, G. Sarosy, J. Plowman, DF Hoth, B. Leyland-Jones: N-methylformamide: cytotoxic, radiosensitizer, or chemosensitizer. In: Journal of clinical oncology: official journal of the American Society of Clinical Oncology. Volume 6, Number 5, May 1988, pp. 906-918, ISSN 0732-183X , PMID 3284976 , (Review).
- ↑ CM Arundel, CM Vines, PJ Tofilon: Chromatin modifications associated with N-methylformamide-induced radiosensitization of clone A cells. In: Cancer Research . Volume 48, Number 20, October 1988, pp. 5669-5673, ISSN 0008-5472 , PMID 3167825 .
- ↑ HF Liao, CD Kuo, YC Yang, CP Lin, HC Tai, YY Chen, YJ Chen: Resveratrol enhances radiosensitivity of human non-small cell lung cancer NCI-H838 cells accompanied by inhibition of nuclear factor-kappa B activation. In: Journal of radiation research. Volume 46, Number 4, December 2005, pp. 387-393, ISSN 0449-3060 , PMID 16394628 .
- ^ R. Pruitt, N. Sasi, ML Freeman, KR Sekhar: Radiosensitization of cancer cells by hydroxychalcones. In: Bioorganic & medicinal chemistry letters. Volume 20, Number 20, October 2010, pp. 5997-6000, ISSN 1464-3405 . doi : 10.1016 / j.bmcl.2010.08.081 , PMID 20826087 . PMC 2946792 (free full text).
- ↑ D. Francis, GM Richards, A. Forouzannia, MP Mehta, D. Khuntia: Motexafin gadolinium: a novel radiosensitizer for brain tumors. In: Expert Opinion on Pharmacotherapy . Volume 10, Number 13, September 2009, pp. 2171-2180, ISSN 1744-7666 . doi : 10.1517 / 14656560903179325 , PMID 19640206 .
- ↑ D. Magda, C. Lepp, N. Gerasimchuk, I. Lee, JL Sessler, A. Lin, JE Biaglow, RA Miller: Redox cycling by motexafin gadolinium enhances cellular response to ionizing radiation by forming reactive oxygen species. In: International journal of radiation oncology, biology, physics. Volume 51, Number 4, November 2001, pp. 1025-1036, ISSN 0360-3016 , PMID 11704327 .
- ^ S. Xu, K. Zakian, H. Thaler, C. Matei, A. Alfieri, Y. Chen, JA Koutcher: Effects of Motexafin gadolinium on tumor metabolism and radiation sensitivity. In: International journal of radiation oncology, biology, physics. Volume 49, Number 5, April 2001, pp. 1381-1390, ISSN 0360-3016 , PMID 11286846 .