Sarin
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||
| 1: 1 mixture (racemate) of the enantiomers : ( R ) form (left) and ( S ) form (right) | |||||||||||||
| General | |||||||||||||
| Surname |
|
||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 4 H 10 FO 2 P | ||||||||||||
| Brief description |
colorless to yellow-brown, odorless liquid |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 140.09 g mol −1 | ||||||||||||
| Physical state |
liquid |
||||||||||||
| density |
1.09 g cm −3 |
||||||||||||
| Melting point |
−57 ° C |
||||||||||||
| boiling point |
147.3 ° C with partial decomposition |
||||||||||||
| Vapor pressure |
197 Pa (20 ° C) |
||||||||||||
| solubility |
|
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| Toxicological data | |||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Sarin is a chemical warfare agent from the group of phosphonic acid esters . The systematic name is isopropyl methyl fluorophosphonate . The common name sarin was introduced in 1943. Sarin was the second in a series of four organophosphorus compounds with potential as chemical warfare agents that were synthesized by IG Farben in the 1930s and 1940s . The others were Tabun (1936), Soman (1944), and Cyclosarin (1949).
history
From insecticide research to chemical weapons
Like Tabun (1936), sarin was discovered in the course of insecticide research in 1939 by a research group led by chemist Gerhard Schrader ( IG Farben in Leverkusen).
In 1934 Gerhard Schrader was commissioned to develop pesticides that were not dependent on imports. Due to the already known, highly toxic aliphatic fluorocarbon compounds (fluorocarboxylic acids and derivatives, fluoroalcohols), the element fluorine, which had been neglected until then, attracted attention. From 1934 the systematic investigation of organic acid fluorides for their suitability as pesticides began (first sulfonic acid fluorides, later fluorides of organic phosphoric acids). One of the first substances examined was methanesulfonic acid fluoride, which was synthesized by Davies and Dick in England in 1932. This highly toxic compound was soon discarded as it is strongly absorbed by grain and food and poisoned them for a long time. In addition to methanesulfonic acid fluoride, aminosulfonic acid fluorides such as dimethylaminosulfonic acid fluoride, which has a weaker insecticidal effect, were also investigated.
At the same time, Schrader was also working on plasticizers based on organic phosphorus compounds. The synthesized substances (esters and amides of phosphoric acid) were also tested by Kükenthal for a possible insecticidal effect for the sake of completeness. Numerous substances unexpectedly turned out to be extremely effective. The systematic investigation of organic phosphorus compounds was then tackled. In analogy to methanesulfonic acid fluoride and the aminosulfonic acid fluorides, methanephosphonic acid (ester) fluorides and aminophosphoric acid (ester) fluorides were also investigated. Of the compounds initially prepared, dimethylaminophosphoric acid difluoride (as a P-analogue to dimethylaminosulfonic acid fluoride) proved to be only weakly insecticidal. In contrast, the dimethylaminophosphoric acid ethyl ester fluoride (fluorine tabun) showed a very strong effect. In 1936, replacement of the fluorine atom with a cyano radical led to the even more toxic dimethylaminophosphoric acid ethyl ester cyanide ( Tabun , Trilon 83, T 83). Tabun was the first compound of the so-called “Trilone”, which aroused considerable interest in specialist circles due to its unexpectedly high toxicity and was reported to the Army Weapons Office.
Methane phosphonic acid (ester) fluorides have also been produced as P analogs to methanesulfonic acid fluoride. In 1938 Schrader synthesized the methanephosphonic acid ethyl ester fluoride under the experiment number 113. Systematic structural modifications then led in 1939 to the synthesis of the far more toxic isopropyl methanephosphonate fluoride (T 144, Trilon 144, later T 46, Trilon 46, Sarin). Sarin proved to be an extraordinarily strong poison, the toxicity of which exceeded all compounds produced up to that point in its warm-blooded animals and was 3–4 times more toxic than tabun. This connection was also reported to the Army Weapons Office. The methane phosphonic acid isopropyl ester fluoride was given the code name Sarin, which was formed from letters of the names of the people involved in the discovery and large-scale technical development: S chrader, A mbros , R itter and von der L in de (the head of the "Heeresgasschutzlaboratoriums" in Spandau , where the Development was going on). In the older literature there is the false claim that instead of the chemist Gerhard Ritter ( Reichsamt für Wirtschaftsausbau ), Colonel Rüdiger from the gas protection department (Wa Prüf 9) in the Army Weapons Office was one of the namesake - without citing the source . This has been corrected in recent research.
The systematic structural modifications led to the synthesis of methanephosphonic acid pinacolyl ester fluoride ( soman ) in 1944 , the toxicity of which was about three times as high as that of sarin. In contrast to Tabun and Sarin, Soman was not developed by Gerhard Schrader, but by Nobel Prize winner Richard Kuhn and his colleague Konrad Henkel . Soman was a product of chemical weapons research; Tabun and sarin were not purely military-chemical developments.
The chosen collective name “Trilone” is said to have been used to mislead, as textile and dyeing aids were also on the market under this name. These were Trilon A (based on the sodium salts of nitrilotriacetic acid) and Trilon B (based on the sodium salts of ethylenediaminetetraacetic acid). Research in this area continued after the war. In 1949, methane phosphonic acid cyclohexyl ester fluoride ( cyclosarin ) was produced.
Sarin is structurally similar to the pesticides parathion (E605) and malathion and also to the warfare agents tabun, soman and VX . In July 1944, 30 tons of sarin were produced in German test factories; but these were never used in combat. Two large plants for mass production were under construction in Germany at the end of the Second World War . In addition to the plant in Dyhernfurth , which was mainly used for tabun production, it was decided in 1943 to build a new plant in Falkenhagen ( Falkenhagen bunker ). The reason was that while sarin was more difficult to make than tabun, it was better as a chemical weapon (it was more toxic and volatile). The supplies in the Dyhernfurth production facility in Silesia fell into the hands of the Red Army at the end of the war in 1945 . The latter had already learned about sarin through espionage in 1943 and synthesized it during the war in Kazan under the direction of Alexander Arbusow .
After the Second World War
Large quantities of sarin were stored in the United States and the Soviet Union during the Cold War . The UK admitted in 2003 that a Royal Air Force soldier had died while experimenting with sarin on humans in their poison gas laboratory in Porton Down , Wiltshire, in 1953 .
During the dictatorship under Augusto Pinochet , the chemist Eugenio Berríos produced DINA Sarin for the Chilean secret service , which was then also used against opposition members.
The Iraq began in 1988 may sarin against its Kurdish minority one ( poison gas attack on Halabja ). Up to 5000 Kurds died in Halabja.
Sarin was also used in two terrorist attacks by the so-called Aum sect (Japanese Ōmu Shinrikyō ), in Matsumoto in 1994 and in Tokyo in 1995 . 20 people were killed and hundreds injured.
In the Syrian civil war Sarin was used several times. For the first time, Sarin could be materially proven as the cause of death in a woman who died on April 29, 2013 near Sarakeb .
Sarin was also used in the Ghouta poison gas attacks on August 21, 2013 . In an attack on Chan Schaichun also Sarin was used on April 4, 2017 at 6:30 am, the Organization for the Prohibition of Chemical Weapons (OPCW) in its report of 29 June 2017. The use of sarin and chlorine gas in Ltamenah on On March 24th and 25th, 2017, the OPCW confirmed in June 2018.
properties
Pure sarin is a colorless, almost odorless, relatively volatile liquid. It can be mixed with water in any ratio. In water, sarin decomposes depending on the pH value : at pH 7, the half-life of the hydrolysis of the ester is about 100 to 150 hours; In acidic solution the same amount decomposes in two hours, in alkaline solution in one hour. Technical sarin can sometimes be yellowish to brownish in color due to impurities and have a slightly fruity odor. In its highly pure form, however, it is almost odorless. Depending on the purity, the odor is described as a typical weak “ester odor”, which is supposed to be reminiscent of highly diluted ethyl acetate. In a series of tests, the odor could be perceived from a concentration in the range of around 1.5 mg / m 3 , although the odor could not be defined by the test persons at this concentration. The test persons also stated that odor recognition is unlikely. For comparison: Soman is said to have a stronger odor at the same concentration, which was individually described as camphor-like, musty and dull, spicy or even fruity.
In numerous human studies with sublethal doses of sarin, its effects on humans under a wide variety of conditions have been examined in detail. Accidents while handling sarin showed that inhaling high concentrations of sarin is extremely dangerous and can lead to unconsciousness and cramps within a few seconds, followed by respiratory arrest 1–2 minutes later. At lower concentrations, on the other hand, symptoms of poisoning develop much more slowly.
Mode of action

Sarin, Acetylcholinesterase, Acetylcholine
Nerve agents like sarin are lethal in very small amounts. The target area is the entire body, with absorption taking place in particular through the eyes , skin and respiratory organs ; The latter make up the main part, since sarin is highly volatile . Protection against the penetration of sarin into the body is therefore only offered by a full-body protective suit with a respirator .
The poisonous effect of sarin is based on an intervention in the transmission of excitation in the nerve tracts: an excitation is transmitted between two nerve cells by a neurotransmitter , which is transmitted through the synaptic gap from the "sender cell" ( presynaptic ending ) to the receptors of the "recipient cell " (postsynaptic region) arrives and thus passes the excitement on to the latter. Often the neurotransmitter is acetylcholine . Immediately after it has been released into the synaptic gap , the acetylcholine is broken down by the enzyme acetylcholinesterase , whereby the excitation is terminated and the recipient cell is available for the next transmission of excitation.
Sarin blocks the acetylcholine esterase in all synapses of the parasympathetic autonomic nervous system , in the acetylcholine-mediated synapses of the sympathetic and on the neuromuscular or motor endplates . This increases the acetylcholine level in the synaptic gap and all affected nervous systems are permanently excited.
Depending on the severity of the poisoning , the following symptoms occur: runny nose, visual disturbances, pupil constriction , eye pain, shortness of breath , salivation , muscle twitching , cramps , sweating, vomiting , uncontrollable stool , unconsciousness , central and peripheral respiratory paralysis and death. The effect on the eye occurs even at lower concentrations than the effect in the respiratory tract , so that accommodation disorders and constriction of the pupils can already be observed at concentrations and exposure times at which the other signs of intoxication are not yet noticeable.
Since the sarin, like other cholinesterase inhibitors, cannot be released from the blocked enzyme, or only very slowly, the treatment of poisoning with such warfare agents is extremely difficult.
Effects similar to those of sarin can also be seen with the chemically related warfare agents tabun, soman and VX as well as with poisoning with various insecticides such as parathion (E605), with sarin being around 1000 times more effective and thus more toxic than E605. The British warfare agent researcher Saunders pointed out at the time that the splitting off of the fluorine atom leads to a decrease in the toxicity of sarin. According to Saunders, dehydrofluorinated isopropylmethylphosphonic acid (IMPA) is a "non-toxic acid". This is also underlined by analyzes of the well-known sarin degradation products IMPA, methylphosphonic acid (MPA), diisopropylmethylphosphonic acid (DIMP), fluoride and methylphosphonyl difluoride. IMPA, MPA and DIMP show low toxicity in short and long term studies.
Protective measures and decontamination
Extensive freely accessible information is available on general protective measures, signs of the use of chemical warfare agents such as exposure to sarin, and decontamination.
Before using warfare agents, oxime tablets or carbamates such as pyridostigmine or physostigmine can be taken. In the case of poisoning, one injects atropine (see hyoscyamine , poison of the deadly nightshade), a parasympatholytic that is supposed to neutralize the effect of the excess acetylcholine at the receptors. In the course of the weeks of follow-up treatment, one can try to regenerate the acetylcholinesterase with an oxime . Obidoxime is preferred in German-speaking countries, while pralidoxime is preferred in Anglo-American countries .
For decontamination, both oxidizing agents such as chlorinated lime or calcium hypochlorite and alkaline solutions, but also non-aqueous media such as ethanolamine, can be used for decontamination, as nerve agents are sensitive to oxidizing agents and are easily hydrolyzed in a basic environment . Sodium carbonate solution can also be used on sensitive surfaces, but this naturally works more slowly.
Another possibility for decontamination is the use of suitable enzymes , which bring about rapid hydrolysis of this and other G-series warfare agents. One of these enzymes is DFPase ( diisopropyl fluorophosphatase , EC 3.1.8.2 ), an enzyme from the common squid Loligo vulgaris . The natural benefits of the enzyme are so far unknown. 105 µg of sarin are completely hydrolyzed in situ within 20 minutes .
Structure and manufacture
Sarin has a stereocenter on the phosphorus atom, so there are two enantiomers , one has an ( R ) configuration, the other has an ( S ) configuration. The manufacturing processes described here produce a racemic sarin, that is to say a 1: 1 mixture of the ( R ) -methylfluorophosphonic acid isopropyl ester and the ( S ) -methyl fluorophosphonic acid isopropyl ester.
By the action of methyl iodide ( 2 ) is removed from the phosphorous acid ester ( 1 () Diisopropylfluorphosphit ) in a Phosphonatsynthese the fluorine methyl phosphonic acid isopropyl ester Sarin ( 3 ) (+ 2-iodopropane ( 4 )) are prepared:
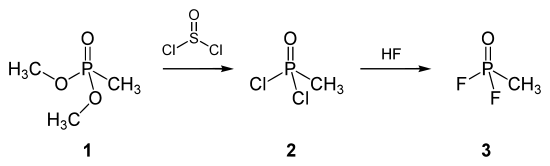
The American method of making sarin is based on the use of dimethyl methylphosphonate ( 1 ). This is converted with thionyl chloride to methylphosphonic acid dichloride ( 2 ) which, after fluorination with hydrofluoric acid , reacts to methylphosphonic acid difluoride ( 3 ):
The methylphosphonic acid difluoride can finally be converted to sarin by adding isopropanol :
When used in binary warfare projectiles, the last reaction above is used, in that methylphosphonic acid difluoride and isopropanol react to sarin after the grenades have been fired - with the aid of a reaction accelerator; the end product is formed after 10 seconds with a 70% yield.
Stereochemistry
The optical isomers of sarin were obtained from enantiomerically pure O -isopropyl-methylphosphonthiolic acid sodium salt [MeP (O) (SNa) (O i Pr)] and picryl fluoride (2,4,6-trinitrofluorobenzene) in methyl acetate ( acetone is less favorable as a solvent , since small amounts of diacetone alcohol that are difficult to separate by distillation are formed here). Optically active sarin is unstable in its pure form and racemizes completely within 20 hours at room temperature. Fluoride ions (e.g. in the form of ammonium fluoride ) catalyze the racemization very strongly. Rapid racemization also takes place in aqueous buffered solution (pH 4.5) . In contrast, dilute solutions of sarin (0.1–0.14 mol / l) in dry isopropanol, acetone or methyl acetate are stable for several weeks.
The optical isomers differ greatly in their toxicity. The main active enantiomer is (-) - sarin, which is around two times more toxic than (±) - sarin, while (+) - sarin is rapidly enzymatically degraded by the sarinase. The table shows the toxicological data of the two enantiomers [( S ) -sarine and ( R ) -sarine] and of the racemate [1: 1 mixture of ( S ) -sarine and ( R ) -sarine] and for comparison corresponding values of Tabun and VX under analogous conditions.
Toxicity of sarin, tabun and VX in mice after intravenous administration:
| Substance or isomer | LD 50 (mouse, µg / kg, iv) |
|---|---|
| (±) -Sarin [( RS ) -Sarin] | 83 |
| (-) - Sarin [( S ) -Sarin] | 41 |
| (+) - Sarin [( R ) -Sarin] | not available |
| (±) -Tabun [( RS ) -Tabun] | 208 |
| (-) - Tabun [( S ) -Tabun] | 119 |
| (+) - Tabun [( R ) -Tabun] | 837 |
| (±) -VX [( RS ) -VX] | 20.1 |
| (-) - VX [( S ) -VX] | 12.6 |
| (+) - VX [( R ) -VX] | 165 |
Analytics
The substance can be reliably identified by suitable sample preparation and subsequent gas chromatography or high-performance liquid chromatography coupled with mass spectrometry . Both urine and blood samples can be used to reliably detect exposure to sarin. As a rule, the metabolites such as B. the alkyl methylphosphonic acids isolated with adequate sample preparation and optionally derivatized for GC-MS analysis .
destruction
The safe and reliable destruction of chemical warfare agents such as sarin and the like is tied to expensive and time-consuming procedures. The main methods apply hydrolytic and / or catalytic processes, mostly at high temperatures and with the use of strong oxidizing agents such as e.g. B. hydrogen peroxide .
International controls
As a chemical on list 1 in the international disarmament treaty CWC, sarin is controlled by the competent authority, the Organization for the Prohibition of Chemical Weapons (OPCW), based in The Hague . Manufacture or possession is prohibited; This does not apply to work that only serves to protect against these substances or for research. In Germany, any non-governmental handling of sarin must be approved by the Federal Office of Economics and Export Control (BAFA) and reported to the OPCW.
See also
Web links
- Factsheet Sarin (SPIEZ LABORATORY) (PDF; 65 kB)
Individual evidence
- ↑ a b c d e Entry on sarin in the GESTIS substance database of the IFA , accessed on December 27, 2019(JavaScript required) .
- ^ Brockhaus ABC Chemie , VEB F. A. Brockhaus Verlag Leipzig 1965, p. 1230.
- ^ S. Franke: Development of chemical warfare, chemistry of warfare agents. 2nd Edition. Military publishing house of the GDR, Berlin 1977 ( textbook of military chemistry. Volume 1).
- ↑ a b G. Hommel: Handbook of dangerous goods. Transport and hazard classes. Volume 6 Springer, Berlin Heidelberg 2012, ISBN 978-3-642-25051-4 (leaflet 2282 and 2002).
- ↑ a b c d Entry on sarin in the ChemIDplus database of the United States National Library of Medicine (NLM)
- ^ R. H. Rengstorff: Accidental exposure to sarin: vision effects. In: Archives of Toxicology . 56, No. 3, 1985, pp. 201-203 ( doi: 10.1007 / BF00333427 ).
- ^ Science Journal. Vol. 155, Issue 3760, 1967, pp. 299-303, doi: 10.1126 / science.155.3760.299 .
- ^ The German health system. Vol. 15, 1960, p. 2179.
- ^ British Journal of Pharmacology . Vol. 39 (4), 1970, p. 822, doi: 10.1111 / j.1476-5381.1970.tb09909.x .
- ↑ a b K. Lohs: Synthetic poisons . Fourth, revised and expanded edition. Military publishing house of the German Democratic Republic, Leipzig 1974.
- ^ R. Harris , J. Paxman: A higher form of killing. The secret history of B and C weapons. Econ, Düsseldorf 1983, ISBN 978-3-430-14052-2 , p. 75.
- ↑ On the other hand, it is named after G. Ritter , a chemist responsible for chemical weapons production in the Reich Office for Economic Development as part of the four-year plan . Compare the report by G. Schrader : History of the development of new insecticides. Part 2: Organic phosphoric acid compounds. October 30, 1945, p. 21, National Archives Washington, RG 319, Entry IRR, Box 200, cit. in: F. Schmaltz: Warfare agent research in National Socialism. For cooperation between Kaiser Wilhelm Institutes, the military and industry. Göttingen 2005, p. 448. For the changing camouflages see ibid.
- ^ S. Everts: The Nazi origins of deadly nerve gases. In: Chemical and Engineering News October 17, 2016.
- ↑ H. Sietz: Sarin chemical weapon, a German invention. In: Zeit Online. June 27, 2013.
- ^ A. Barnett: Final agony of RAF volunteer killed by sarin - in Britain , In: theguardian.com , September 28, 2003.
- ↑ C. Seidler: Poison gas Sarin. Deadly constant stress. In: Spiegel Online . April 26, 2013.
- ↑ SRF : 37 years after the coup in Chile: condemned by the military. September 11, 2010.
- ↑ H. John, MJ van der Schans, M. Koller, HET Spruit, F. Worek, F. Thiermann, D. Noort: Fatal sarin poisoning in Syria 2013: forensic verification within an international laboratory network, Forensic Toxicology, Volume 36, 2018, pp. 61–71, doi: 10.1007 / s11419-017-0376-7
- ^ R. Gladstone, N. Cumming-Bruce: UN Report Confirms Rockets Loaded With Sarin in Aug. 21 Attack. In: The New York Times . 16th September 2013.
- ^ Report of the OPCW Fact - Finding Mission in Syria regarding an alleged Incident in Khan Shaykhun, Sryian Arab Repbulic. (PDF) June 27, 2017, accessed June 27, 2017 .
- ^ Organization for the Prohibition of Chemical Weapons : OPCW Confirms Use of Sarin and Chlorine in Ltamenah, Syria, on 24 and 25 March 2017. June 13, 2018, accessed on March 12, 2019 .
- ^ Federal Office for Civil Protection Labor Spiez: Sarin. (PDF; 51 kB), accessed on February 4, 2017.
- ↑ a b S. Eckert: Development of a dynamic model to study the protective effects of reversible acetylcholinesterase inhibitors against irreversible inhibition by highly toxic organophosphates. (PDF; 1.3 MB) Dissertation . University of Munich, 2006, p. 2.
- ↑ B. C. Saunders (Ed.): Some aspects of the chemistry and toxic action of organic compounds containing phosphorus and fluorine. Cambridge University Press, Cambridge 1957, pp. 93 .
- ↑ K.K. Kroening, R. N. Easter, D. D. Richardson, St. A. Willison, J. A. Caruso: Analysis of Chemical Warfare Degradation Products, Chapter 2, Toxicity of Chemical Warfare Agents and their Degradation Products, Wiley-Blackwell, 2011, pp. 19-57
- ↑ K. L. Koenig: Preparedness for terrorism: managing nuclear, biological and chemical threats. In: Ann. Acad. Med. Singapore. 38 (12), 2009, pp. 1026-1030. PMID 20052435 .
- ↑ L. Szinicz, S. I. Baskin: Chemical and biological warfare agents. In: Textbook of Toxicology. 2nd edition, W. V., Stuttgart 2004, ISBN 978-3-8047-1777-0 , pp. 865-895.
- ↑ cf. Improved detoxification solution EP 1802377 B1. last accessed April 25, 2013.
- ^ A. Richardt, M. Blum, St. Mitchell: What Do Calamari Know About Sarin? Enzymatic decontamination of nerve warfare agents. In: Chemistry in Our Time . 40, No. 4, 2006, pp. 252-259 ( doi: 10.1002 / ciuz.200600364 ).
- ^ Entry on Sarin. In: Römpp Online . Georg Thieme Verlag, accessed on September 9, 2013.
- ↑ a b H. L. Boter, A. J. J. Ooms, G. R. Van DenBerg, C. Van Dijk: The synthesis of optically active isopropylmethylphosphonofluoridate (sarin). In: Recueil des Travaux Chimiques des Pays-Bas . tape 85 , 1966, pp. 147-150 .
- ↑ H. P. Benschop, L. P. A. De Jong: Nerve agent stereoisomers: analysis, isolation and toxicology. In: Acc. Chem. Res. Volume 21 , no. 10 , 1988, pp. 368-374 , doi : 10.1021 / ar00154a003 .
- ↑ G. L. Hook, C. Jackson Lepage, S. I. Miller, P. A. Smith: Dynamic solid phase microextraction for sampling of airborne sarin with gas chromatography-mass spectrometry for rapid field detection and quantification. In: J Sep Sci . 27 (12), August 2004, pp. 1017-1022, PMID 15352721 .
- ↑ Shaner RL, Coleman RM, Schulze N, Platanitis K, Brown AA, Seymour C, Kaplan P, Perez J, Hamelin EI, Johnson RC: Investigation of dried blood sampling with liquid chromatography tandem mass spectrometry to confirm human exposure to nerve agents. , Anal Chim Acta. 2018 Nov 29; 1033: 100-107, PMID 30172315
- ↑ W. J. Driskell, M. Shih, L. L. Needham, D. B. Barr: Quantitation of organophosphorus nerve agent metabolites in human urine using isotope dilution gas chromatography-tandem mass spectrometry. In: J Anal Toxicol . 26 (1), January-February 2002, pp. 6-10, PMID 11888020 .
- ↑ M. Nagao, T. Takatori, Y. Matsuda, M. Nakajima, H. Iwase, K. Iwadate: Definitive evidence for the acute sarin poisoning diagnosis in the Tokyo subway. In: Toxicol Appl Pharmacol . 144 (1), May 1997, pp. 198-203, PMID 9169085 .
- ↑ B. Veriansyah, E. S. Song, J.D. Kim: Destruction of methylphosphonic acid in a supercritical water oxidation bench-scale double wall reactor. In: J Environ Sci (China). 23 (4), 2011, pp. 545-552. PMID 21793394 .
- ↑ E. Gershonov, I. Columbus, Y. Zafrani: Facile hydrolysis-based chemical destruction of the warfare agents VX, GB, and HD by alumina-supported fluoride reagents. In: J Org Chem. 74 (1), Jan. 2, 2009, pp. 329-338. PMID 19053582 .
- ↑ Federal Office of Economics and Export Control (BAFA): List 1 chemicals , accessed on September 4, 2013.
- ↑ Federal Office of Economics and Export Control (BAFA): Chemical Weapons Convention , accessed on September 4, 2013.