Weakly coordinating ions
In chemistry , weakly coordinating ions refer to ions that only interact weakly with other molecules or ions. The strong electrostatic interactions between cations and anions are replaced by a number of weaker interactions. These interactions relate primarily to the formation of coordinative bonds . Weakly coordinating ions are often large molecules with diameters in the nanometer range.
The solubility of salts from weakly coordinating ions in slightly or non-polar solvents is higher than that of classical salts; the reduced tendency to form ion pairing can contribute to electrical conductivity in these solvents. To emphasize that the ions are largely independent of one another, the terms free ion or naked ion are also used. Such ions have long been known in the gas phase . Weakly coordinating ions are increasingly being used to produce compounds that have comparable properties in solution or in the solid . Weakly coordinating ions are of increasing importance as they enable the investigation of highly reactive compounds using a variety of physical and chemical methods. Weakly coordinating ions are used in practice, for example, in the production of novel catalysts , in coordinative polymerization , in the development of ionic liquids as solvents for chemical reactions and in electrochemistry .
history
In the 1970s, complex anions such as BF 4 - , ClO 4 - or anions of hexahalogenated non- or semimetals of the nitrogen group such as [SbCl 6 ] - were regarded as non- coordinating anions. However, through crystal structure analysis it was recognized that the noncoordinating anions present in aqueous solution do coordinate in the solid body when the solvent is removed.
In order to describe the occurrence of coordination of complex anions, the term "weakly coordinating anions" was coined. These were regarded as the starting point for the development of increasingly weaker coordinating anions with the ultimate goal of a non-coordinating anion that could not be achieved.
A milestone was reached in the 1990s with the synthesis of the tetrakis [3,5-bis (trifluoromethyl) phenyl] borate ion ([B [3,5- (CF3) 2 C 6 H 3 ] 4 ] - ). This anion coordinated much more weakly than the previously known weakly coordinating anions and allowed the study of strongly electrophilic cations.
Basics
Coordinative bond
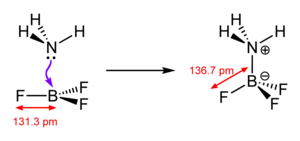
A coordinative bond describes a chemical bond in which the binding electrons are only provided by one binding partner. The best-known representatives of these compounds are ionic complexes . Several negatively charged anions are grouped around a positively charged cation . The anions use a lone pair of electrons to bind as a ligand to the cation, the central atom . The number of surrounding ions is the coordination number and the spatial arrangement is represented by the coordination polyhedron .
In the solid state, ions are arranged in an ion lattice . Both cations and anions are surrounded by several oppositely charged particles (the counterions ).
A "weak" coordination in this context means that the binding energy of the coordinative bond is very low. Since the anion always contributes the binding electrons, the coordination ability mostly depends on the nature of the anion. It is possible, especially in solids and melts , to influence the strength of the bond through the properties of the cation.
Free ions in the gas phase
In vacuo ions generated are due to the large spatial distances to all the other atoms as free-floating in space charge carriers . They are often generated in an ion source by targeted bombardment with electrons ( impact ionization ) or charge transfer through another ionized gas ( chemical ionization ) and are mainly investigated by mass spectrometry .
In the food industry, for example, ionized air is used to pasteurize beverages. The high reactivity of the ions is used here. However, this fact shows that such ions usually only have a very short lifespan and practically decay or continue to react immediately after they are generated. As a result, it is not possible to carry out lengthy spectroscopic investigations ( NMR , IR , Raman , UV / VIS ). Due to the restriction to the gas phase, diffraction experiments such as X-ray diffraction or neutron scattering are impossible.
Free ions in solutions and solids
The definition of liquid and solid states of aggregation requires that particles always interact with one another. Therefore there can be no “free” ions in these states.
Influence of the solvent
In solutions, ions are surrounded and solvated by the solvent . The solvent acts as a dielectric (insulator) in that it is reversed to the charge of the ion and thus weakens the electrical field around the ion. The measure for this weakening is the polarity of the solvent, which is expressed by its dielectric constant (ε r ).
In a strongly polar solvent such as water (ε r = 80), dissolved ions hardly interact with one another. However, the interactions with the solvent are all the stronger, which can be illustrated by the example of lithium ions: Due to its large hydrate shell , Li + shows a much lower mobility than the much larger sodium or potassium ions.
When switching to non-polar solvents such as dichloromethane (ε r = 9) or diethyl ether (ε r = 4.3), it becomes apparent that many ions coordinate strongly with one another, which is mainly expressed in the fact that most salts are insoluble in such solvents : They form strong bonds in the form of a crystal lattice .
Ions in solids
In the solid state, the measure of the strength of the interactions between the ions is the lattice energy . The greater the distance between oppositely charged ions, the smaller the lattice energy. This can be demonstrated using the following table:
| Surname | formula |
Ionic radius of the monovalent alkali metal cations X + in pm |
Lattice enthalpy in kJ per mol |
|---|---|---|---|
| Lithium fluoride | LiF | 74 | 1039 |
| Sodium fluoride | NaF | 102 | 920 |
| Potassium fluoride | Theatrical Version | 138 | 816 |
| Rubidium fluoride | RbF | 149 | 780 |
| Cesium fluoride | CsF | 170 | 749 |
In the transition from monatomic to polyatomic ions, the ionic radius loses its informative value , since only very few molecular ions have a similarly highly symmetrical structure as individual atomic ions. Especially in the case of rather small but very asymmetrical ions, the lattice energies calculated with the help of the ion radii deviate widely from the experimental values. To take this fact into account, Donald Jenkins introduced the concept of thermochemical volume .
The volume of an ion is calculated from the volume of the unit cell of an ion crystal and the lattice energy is determined from this. As a semi-empirical method, the calculated lattice energies agree in many cases with the experimental values.
Concepts
The approach to developing weakly coordinating ions is to distribute a small charge over the largest possible volume. This minimizes the lattice energy in the solid (and thus the interaction between oppositely charged ions). In addition, the ion must have a low polarizability so that a nearby counterion or solvent particles cannot generate centers of charge. Otherwise, these would in turn cause dipole-dipole forces and thus lead to coordination.
The easy polarizability is the reason why large single-atom ions such as iodide or cesium only have a limited effect as weakly coordinating ions. Research today therefore focuses on the production of very large monovalent (simply positively or negatively charged) molecules.
Weakly coordinating anions

Weakly coordinating anions are mainly realized by two different methods.
Covalently bound framework anions
One possibility is the construction of a polyatomic, negatively charged framework, which has a surface as spherical as possible on which the charge is distributed. The atoms of the framework are held together by strong covalent bonds .
The main representatives of this class are negatively charged carboranes such as [CB 11 H 12 ] - . By substituting all H atoms, the even more stable carborate [1-R-CB 11 F 11 ] - (R = Me, Et) could be obtained, which is traded as the best weakly coordinating anion to date.
Stable Lewis acid-base complexes
The second approach is the construction of particularly stable complex anions from strong Lewis acids and Lewis bases . A cation with the charge X and X + 1 negatively charged ligands results in a complex with a total charge of −1. A strong coordinative bond between the ligands and the central atom is important for the stability of the complex. Up to now, highly charged cations such as B 3+ , Al 3+ , As 5+ , Sb 5+ , Nb 5+ , Y 3+ or La 3+ have been used for this.
For the formation of a strong bond, atoms with a high electronegativity such as fluorine or oxygen are particularly suitable as ligands . Anions such as tetrafluoroborate ([BF 4 ] - ), perchlorate ([ClO 4 ] - ) or hexafluoroantimonate ([SbF 6 ] - ) are already widely used in industry. However, it has been proven that such anions coordinate comparatively strongly to cations in non-polar solvents.
| Frequently used ligands | ||
|---|---|---|
| designation | Molecular formula | perfluorinated |
| Alkyl | ||
| Methyl- | -CH 3 | -CF 3 |
| t-butyl | -C (CH 3 ) 3 | -C (CF 3 ) 3 |
| Aryl | ||
| Phenyl | -C 6 H 5 | -C 6 F 5 |
| Alkoxy | ||
| Methoxy | -O-CH 3 | -O-CF 3 |
| t-butoxy | -OC (CH 3 ) 3 | -OC (CF 3 ) 3 |
| Aryloxy | ||
| Phenyloxy | -OC 6 H 5 | -OC 6 F 5 |
| Perfluorotelluroxy | ||
| Teflat | -O-TeF 5 | |
In research, therefore, ligands are increasingly being used which have bulky substituents and a chemically inert surface. Important representatives are alkyl and aryl ligands such as [BPh 4 ] - ( Kalignost ). The alkoxy and aryloxy ligands are derived from the corresponding alcohols and are bonded to the central atom via the oxygen.
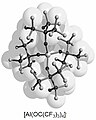
To make the surface of the ions chemically unassailable (inert), perfluorinated versions of the ligands are used (see fluorocarbons and fluorocarbons ). For example, the perfluorinated with tert-butanol ligand anion [Al [OC (CF formed 3 ) 3 ] 4 ] - in its properties comparable with the industrially frequently used [SbF 6 ] - .
- Examples
Weakly coordinating cations
To date, there are hardly any higher-level concepts for the production of weakly coordinating cations. Development mostly focuses on the intended use, for example the stabilization of "naked" fluoride ions and other highly reactive anions.
What weakly coordinating cations have in common is often the build-up of voluminous molecules with positively charged nitrogen , phosphorus or sulfur . The rest of the molecule is constructed in such a way that they are only very weak Brønsted acids , i.e. can not split off protons , which would otherwise lead to the decomposition of the cation. This means that these molecules can be viewed as salts of very strong bases , a property that opens up further possible applications.
The following table lists some cations for which weakly coordinated fluoride compounds have been detected:
Thermodynamic properties
By measurements and calculations with the help of the Born-Haber cycle it is possible to determine thermodynamic properties of compounds with large and weakly coordinating ions. The comparison of the properties for different ions is a measure of the quality of a certain ion.
The calculation of the lattice energy of solid compounds with very large, weakly coordinating anions gives - depending on the volume of the particles - very small values.
| salt | thermochemical volume in Å 3 | Lattice energy U pot. In kJ mol −1 |
|---|---|---|
| Li + F - | 27 | 1036 |
| Cs + F - | 43 | 740 |
| Cs + [AsF 6 ] - | 128 | 568 |
| Cs + [Al {OC (CF 3 ) 4 }] - | 776 | 362 |
| [Ag (S 8 ) 2 ] + [Al {OC (CF 3 ) 4 }] - | 1169 | 326 |
These values can be compared well with the sublimation enthalpies of very heavy molecules (e.g. the fullerenes C 60 and C 70 ):
| salt | Molar mass in g mol −1 | Energy in kJ mol −1 |
|---|---|---|
| [Ag (S 8 ) 2 ] + [Al {OC (CF 3 ) 4 }] - | 1588 | 326 |
| C 70 | 841 | 200 |
| C 60 | 721 | 175 |
The weakly coordinated compounds have energies in the solid state that are comparable to molecules in the gas phase. In fact, the above cation [Ag (S 8 ) 2 ] + was not known prior to being shown with a weakly coordinating anion. Due to the fundamentally low lattice energy, destabilizing effects, such as those caused by very weakly bound ligands (such as sulfur in this case ), are less effective. Conversely, the use of weakly coordinating ions can stabilize such complexes.
Otherwise comparable conditions can partly be achieved by isolating the ions in a matrix or with the aid of slightly modified zeolites .
These low lattice energies result in a number of other properties. Such salts are much more likely to be soluble in non-polar solvents with a low dielectric constant, since the energy barrier to loosening the ionic bond and solvation by the solvent is lower than with conventional salts. In addition, the lattice energy is exceeded in some compounds at room temperature, so that some salts are already liquid at these low temperatures; Table salt, on the other hand, only melts at temperatures above 800 ° C.
Applications
The possible applications are very diverse, so only a few examples are given in this section.
Coordination polymerization
Cationic or anionic coordination polymerization is one of the most important processes for the polymerization of alkenes . For this purpose, positively charged metallocenes with group IV elements such as titanium or zirconium are often used. However, the activity of the catalyst depends strongly on the properties of the counterion. Only when using weakly coordinating ions such as [B (C 6 F 5 ) 4 ] - do the salts formed have the required high activity. This increase in reactivity has been shown to be attributed to the reduced influence of the anion.
A cationic palladium (II) catalyst with a tetrakis [3,5-bis (trifluoromethyl) phenyl] borate anion can be used for the copolymerization of carbon monoxide with ethylene or propylene . The reaction creates polyketones .
Lithium-ion batteries
One of the best-known areas of application within electrochemistry are lithium-ion batteries . In order to achieve a high battery voltage, the choice of the counterion and the solvent is crucial. The weaker the counterion and the more non-polar the selected solvent, the higher the ion mobility of the lithium ions and thus the conductivity of the lithium electrolyte. At present, Li + [PF 6 ] - is used most frequently in this area , but there are already a number of patent applications for the use of other anions.
Ionic liquids
Due to their special properties, ionic liquids open up many possible applications as solvents in organic chemistry, as electrolytes or in catalysis . Based on the very low melting points and extremely low vapor pressures , different solubilities in polar and non-polar solvents and, finally, because of their environmental compatibility, it is to be expected that ionic liquids will displace many organic solvents.
Organic catalysis
In catalytic organic chemistry, highly reactive cations such as Ag + , Li + or F - are sometimes used. The use of weakly coordinating ions enables the reactions to take place at lower temperatures. Fluorination reactions of aromatics, in which, for example, chlorobenzene is converted directly into fluorobenzene, are only possible with very reactive fluoride ions.
literature
- Ingo Krossing, Ines Raabe: Non- coordinating anions - dream or reality? An overview of possible candidates. In: Angewandte Chemie. 116, 2004, pp. 2116-2142, doi : 10.1002 / anie.200300620 .
- AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 .
- E. Riedel (ed.); C. Janiak, TM Klapötke , H.-J. Meyer: Modern inorganic chemistry. Berlin 2007, ISBN 978-3-11-019060-1 (basics of coordination chemistry)
Web links
Individual evidence
- ↑ Michael R. Rosenthal: The myth of the non-coordinating anion. In: Journal of Chemical Education. 50, 1973, p. 331, doi : 10.1021 / ed050p331 .
- ↑ Ingo Krossing, Ines Raabe: Noncoordinating Anions - Dream or Reality? An overview of possible candidates. In: Angewandte Chemie. 116, 2004, pp. 2116-2142, doi : 10.1002 / anie.200300620 .
- ^ Steven H. Strauss: The search for larger and more weakly coordinating anions. In: Chemical Reviews. 93, 1993, pp. 927-942, doi : 10.1021 / cr00019a005 .
- ↑ Neal A. Yakelis, Robert G. Bergman: Safe Preparation and Purification of Sodium tetrakis [(3,5-trifluoromethyl) phenyl] borate (NaBArF 24 ): Reliable and Sensitive Analysis of Water in Solutions of Fluorinated Tetraarylborates. In: Organometallics. 24, 2005, pp. 3579-3582, doi : 10.1021 / om0501428 .
- ^ A b H. Donald B. Jenkins, Helen K. Roobottom, Jack Passmore, Leslie Glasser: Relationships among Ionic Lattice Energies, Molecular (Formula Unit) Volumes, and Thermochemical Radii. In: Inorganic Chemistry. 38, 1999, pp. 3609-3620, doi : 10.1021 / ic9812961 .
- ↑ Daniel Stasko, Christopher A. Reed: Optimizing the Least Nucleophilic anion. A New, Strong Methyl Reagent. In: Journal of the American Chemical Society. 124, 2002, pp. 1148-1149, doi : 10.1021 / ja0118800 .
- ↑ Wolfgang Beck, Karlheinz Suenkel: Metal complexes of weakly coordinating anions. Precursors of strong cationic organometallic Lewis acids. In: Chemical Reviews. 88, 1988, pp. 1405-1421, doi : 10.1021 / cr00089a017 .
- ^ Karl O. Christe, William W. Wilson: Reaction of the fluoride anion with acetonitrile. Chloroform and methylene chloride. In: Journal of Fluorine Chemistry. 47, 1990, pp. 117-120, doi : 10.1016 / S0022-1139 (00) 80453-4 .
- ↑ Andreas Kornath, F. Neumann, H. Oberhammer: Tetramethylphosphonium Fluoride: “Naked” Fluoride and Phosphorane. In: Inorganic Chemistry. 42, 2003, pp. 2894-2901, doi : 10.1021 / ic020663c .
- ↑ Alexander Kolomeitsev, Valery Movchun, Eduard Rusanov, German Bissky, Enno Lork, Gerd-Volker Röschenthaler, Peer Kirsch: Different fluoride anion sources and (trifluoromethyl) trimethylsilane: molecular structure of tris (dimethylamino) sulfonium bis (trifluoromethyl) trimethylsiliconate isolated pentacoordinate silicon species with five Si – C bonds. In: Chemical Communications. , Pp. 1017-1018, doi : 10.1039 / A901953G .
- ↑ Andrew E. Bayliff, Richard D. Chambers: Reactions involving fluoride ion. Part 34. Stable perfluorinated carbanions. In: Journal of the Chemical Society, Perkin Transactions 1. 1988, pp. 201-208, doi : 10.1039 / P19880000201 .
- ↑ Ali Reza Mahjoub, Xiongzhi Zhang, Konrad Seppelt: Reactions of the "Naked" Fluoride Ion: Syntheses and Structures of SeF 6 2- and BrF 6 - . In: Chemistry - A European Journal. 1, 1995, pp. 261-265, doi : 10.1002 / chem.19950010410 .
- ↑ Reinhard Schwesinger, Reinhard Link, Gerhard Thiele, Heinz Rotter, Dieter Honert, Hans-Heinrich Limbach, Ferdinand Männle: Stable phosphazenium ions in synthesis - an easily accessible, extremely reactive “naked” fluoride salt. In: Angewandte Chemie. 103, 1991, pp. 1376-1378, doi : 10.1002 / ange.19911031031 .
- ↑ Eugene You-Xian Chen, Tobin J. Marks: Cocatalysts for Metal-Catalyzed Olefin Polymerization: Activators, Activation Processes, and Structure-Activity Relationships. In: Chemical Reviews. 100, 2000, pp. 1391-1434, doi : 10.1021 / cr980462j .
- ↑ M. Brookhart, Francis C. Rix, JM DeSimone, James C. Barborak: Palladium (II) catalysts for living alternating copolymerization of olefins and carbon monoxide. In: Journal of the American Chemical Society. 114, 1992, pp. 5894-5895, doi : 10.1021 / ja00040a082 .
- ↑ Jeffrey A. Boon, Joseph A. Levisky, J. Lloyd Pflug, John S. Wilkes: Friedel-Crafts reactions in ambient-temperature molten salts. In: The Journal of Organic Chemistry. 51, 1986, pp. 480-483, doi : 10.1021 / jo00354a013 .