Selenium deficiency
| Classification according to ICD-10 | |
|---|---|
| E59 | Alimentary selenium deficiency |
| E64.8 | Consequences of other nutritional deficiencies |
| ICD-10 online (WHO version 2019) | |
If there is a selenium deficiency , the body does not have sufficient amounts of the essential trace element selenium .
Particularly at risk for the occurrence of a relevant selenium deficiency are people who live in areas with pronounced selenium deficiency in the soil, but also vegans and people who are artificially fed over a long period of time .
Selenium deficiency causes a reduced function of selenium-dependent enzymes , which occur in almost all organs, and can therefore cause disorders of various organ systems. For example, the enzymes from the group of glutathione peroxidases , which play an important role in coping with oxidative stress , and certain deiodinases (iodine-removing enzymes), which are important for the effect of thyroid hormones on the body's cells, are selenium-dependent .
The importance of selenium for human metabolic processes was only recognized in the second half of the 20th century, initially only to a limited extent. The first disease that has been unequivocally proven to be caused by a selenium deficiency was Keshan's disease . This is a disease of the heart muscle , in which, however, the presence of certain viruses also plays an important role. Keshan's disease can both be treated and prevented by giving selenium. Other diseases clearly caused by selenium deficiency can also be alleviated by giving selenium.
The scientific results on the effects of selenium deficiency on many organ systems are in some cases still contradicting one another. Some diseases, for which selenium deficiency has not (so far) been identified as the cause, still respond to selenium administration. However, there are no standardized recommendations for the substitution of selenium for the treatment of these diseases. The information on the amounts of selenium healthy individuals should consume daily is also inconsistent.
The opposite of selenium deficiency - an oversupply of selenium - is known as selenosis .
Basics
Selenium enters the food chain by being absorbed from the soil in inorganic form by plants and organically bound. The selenium content of plants does not only depend on its concentration in the soil (acidic soils or those of volcanic origin are poor in selenium ), but is also negatively influenced by the simultaneous presence of other elements (e.g. sulfur , aluminum and iron ).
Selenium balance in humans
Selenium - total content in the human body around 10 to 20 mg - is found in all tissues: around 60% in the kidneys, liver and muscles and around another 30% in the skeletal system . The optimal amount of selenium to supply the human body with this essential trace element is still not known.
Intake and need
The uptake of selenium via the gastrointestinal tract depends on its chemical value in the compound in which it is supplied, not on the state of supply of the body. The intake rate is between 50 and 100%. It is particularly high for sodium selenite (Na 2 SeO 3 ) and somewhat worse for selenomethionine (C 4 H 9 NO 2 Se) and selenocysteine (C 3 H 7 NO 2 Se). The Nutrition Societies in Germany ( DGE ), Switzerland (SGE / SVE) and Austria (ÖGE ) recommend 30 to 70 µg (up to a maximum of 300 µg) as daily intake for adults . In the USA, 55 µg is recommended for women and 70 µg for men, and between 5 and 60 µg daily for children, depending on weight and age. The recommendations are estimated values based on the activity of an enzyme that absolutely needs selenium ( glutathione peroxidase, see below). The activity of glutathione peroxidase in blood serum (GPX 3) reaches its maximum value when the daily intake is 1 µg per kilogram of body weight; the optimal concentration of the transport protein selenoprotein P in the blood serum is only reached at a much higher intake. The occurrence of deficiency symptoms with a long-term intake of less than 10 µg and signs of intoxication ( selenosis ) with over 400–800 µg daily is certain .
Foods rich in selenium are meat, offal (kidney 1200 µg / 100 g, liver 800 µg / 100 g), fish, seafood, milk (30 µg / 1000 g), cheese (60 µg / 100 g), eggs (40 µg / 100 g) g), mushrooms, cereal products (40 µg / 100 g) and legumes. Around 28% of selenium intake comes from meat, especially pork; around 16% from eggs. Regions with particularly low levels of selenium in the soil are parts of Scandinavia, China and New Zealand. The traditional Japanese diet with lots of fish and rice is considered rich in selenium. Brazil nuts have a particularly high selenium content (figures vary between 800 µg / 100 g and 8300 µg / 100 g), in which the selenium also has a high bioavailability.
The assessment of the supply situation in Germany is not presented uniformly in the literature, it ranges from “shortage area” to “secured”. There are indications that around 70% of the population in Germany take in too little selenium ( median 40 µg). According to another source, the mean selenium intake in Germany is 46 µg / day for men and 39 µg / day for women. The intake in the USA is 60 to 200 µg daily.
transport
In the blood, selenium is bound to plasma proteins and transported. In this way it gets into all tissues (including hair and bones). Over 60% of the selenium is bound to the serum glycoprotein selenoprotein P , which is largely produced by the liver (up to 10 atoms per molecule if the supply situation is good ).
storage
Selenium is stored in the body as selenomethionine and mobilized from it when required. Selenocysteine, on the other hand, is the biologically active form. Selenium does not occur unbound in the body. The transport protein selenoprotein P also seems to be of considerable importance for storage.
excretion
The excretion of selenium depends on the selenium status of the body; it occurs increasingly when there is an oversupply. Selenium is primarily excreted in the urine , but also in the stool . Particularly with increased intake, it is also exhaled as dimethyl selenide (C 2 H 6 Se) , which creates a garlic- like odor.
physiology
The human body only incorporates selenium in proteins as selenocysteine . These proteins are then called selenoproteins . Selenocysteine is under healthy conditions in the body in ionized form and thus as an effective redox - catalyst before (significantly more effective than cysteine , the sulfur instead of selenium). As part of the protein folding , it reaches the active center of the respective selenoprotein. The human genome contains 25 genes that contain the genetic information for about 30 to 50 selenoproteins ( technically referred to as “coding for them”) . In some cases, multiple genes code for one protein. The function of 15 of these proteins is at least partially known. From a systematic point of view, the selenoproteins in humans are divided into 17 different groups. In addition to glutathione peroxidases (5 genes code for this), these are thioredoxin reductases (3 genes), deiodases (3 genes) and selenophosphate synthetases. The remaining proteins are named alphabetically as Selenoprotein 15, SelH, SelI, SelK, SelM, SelN, SelO, SelP, SelR, SelS, SelT, SelV and SelW.
The formation of selenoproteins is a complex intracellular process that is fundamentally different from the biosynthesis of other proteins. With the consumption of guanosine triphosphate , the stop codon U - G - A of the messenger RNA (mRNA) serves as a signal for the incorporation of selenium. For this process the SE-CIS-Binding Protein 2 (SBP2), a selenium-specific elongation factor (EFsec) and a transfer RNA (tRNA) loaded with selenocysteine are required. As part of this translation of the mRNA, the amino acid selenocysteine gets into the growing peptide chain.
Glutathione peroxidases
Glutathione peroxidases (GPX 1–7) help reduce oxidative cell and tissue damage by detoxifying unsaturated lipids in the cell membrane . Of the 7 forms known in humans, 5 are selenium-dependent (GPX 1-4 and 6).
In particular from the metabolism of red blood cells known GPX 1 is in the cytosol detected substantially all body cells. Other selenium-dependent glutathione peroxidases can be found, for example, in cells of the gastrointestinal tract (GPX 2) , in blood plasma (GPX 3) , on membrane surfaces of different cells and in the nucleus of sperm (GPX 4) and in olfactory cells (GPX 6) .
The basic function of glutathione peroxidases is that they reduce hydrogen peroxide (H 2 O 2 ) to water (H 2 O) . Thereby oxidize it glutathione .
Peroxides present in body tissues release hydroxyl radicals (OH), which damage cells and tissues. This reaction plays an important role in cell aging and liver damage (caused by alcohol and hydrocarbon tetrachloride ). The reduction of peroxides by glutathione peroxidases is therefore an antioxidant protective mechanism. It is also necessary to maintain the integrity of red blood cells by protecting their membrane lipids from peroxidation.
Deiodase
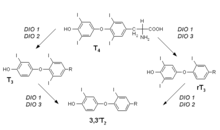
T 4 - thyroxine , T 3 - triiodothyronine , rT 3 - reverse triiodothyronine, 3,3'T 2 - diiodotyrosine , DIO 1–3 - deiodase 1-3
The deiodases are selenium-dependent enzymes that are important for the metabolism of thyroid hormones . Three different forms are currently known (DIO 1–3). Their task is the deiodination (removal of iodine atoms) of the thyroid hormones, which activates them ( thyroxine to triiodothyronine ) or deactivates them (triiodothyronine to diiodotyrosine ). The (type 1) thyroxine-5'-deiodase occurs mainly in the liver, kidneys and muscles. The other deiodases are found, for example, in the CNS and in fetal tissue. The control mechanism underlying the formation of deiodases and in which triiodothyronine and TSH are also involved is still largely unknown. If there is a selenium deficiency, these enzymes are preferentially formed in some tissues (for example, the CNS).
Thioredoxin reductases
Thioredoxin reductases are proteins that play an important role in cell metabolism. They act as catalysts in thiol-disulfide exchange reactions and are important in protein folding ( disulfide bridge formation ) and for the enzyme ribonucleotide reductase , which is required for cell division . They occur both in the cytosol and in the cell nucleus , neutralize reactive oxygen species , regulate redox-sensitive processes and activate transcription factors .
Causes of Selenium Deficiency
Low-selenium foods or an extremely unbalanced diet, but also malabsorption and medication can be the cause of a selenium deficiency. Therefore, not only people who live in selenium-poor areas are at risk, but also vegans and patients who require artificial nutrition . In contrast to vegans, the selenium supply in vegetarians is considered to be secure and is roughly on the same level as in people who eat a mixed diet. The cause may be different absorption rates .
According to the results of a consensus conference of 1997, the following circumstances bring a high risk of selenium deficiency due to decreased intake: pure vegetarianism (vegans), extremely unbalanced diet (eg alcoholics ), diet with tube feedings , parenteral nutrition , dialysis , starvation , anorexia nervosa or bulimia . A risk of selenium deficiency due to increased losses exists with long-term diarrhea , maldigestion or malabsorption (certain digestive disorders), abuse of laxatives (abuse of laxatives), proteinuria in certain kidney diseases, nephrotic syndrome , negative nitrogen balance , diabetes insipidus , treatment with diuretics (water tablets) , heavy bleeding with hemorrhoids or heavy menstrual bleeding , prolonged breastfeeding , severe burns or other injuries .
Pathophysiology
Selenium-dependent enzymes are formed in smaller quantities when there is a selenium deficiency.
In the context of genetic defects , disturbances of selenium-dependent enzymes can also occur. In these cases, the activity of the affected enzymes is measurably reduced even with sufficient intake of the essential trace element. Such genetic defects “simulate” a selenium deficiency. This is used within the framework of basic clinical research to better understand the importance of selenium for the occurrence of pathological changes.
There are indications that when there is a selenium deficiency, the brain , endocrine glands and gonads are given priority. In addition, the body seems to redistribute selenium from glutathione peroxidase 1 (GPX 1) and deiodase 1 (thyroxine-5'-deiodase) in phases of deficiency. In the course of inflammation or infectious diseases, selenium is redistributed from the blood to other areas of the body (e.g. muscles). The cause, meaning and effects of this phenomenon are still unclear.
The glutathione peroxidases seem to be responsible for the prevention of diseases in different ways, depending on the type. Animal experiments have shown, for example, that GPX 1 is important for the defense against viral infections and the prevention of the development of malignant tumors , and that a lack of GPX 2, especially in the gastrointestinal tract, leads to increased bacterial infections and malignant changes, and triggers short stature GPX 4 plays an essential role in sperm production . Changes in the deiodase affect the metabolism of the thyroid hormones and thus also the regulation of body temperature , growth, hearing and especially the development of the brain in the unborn child.
Clinical picture
Different organ systems can be affected in different ways by selenium deficiency. The diseases are ultimately based on the reduced functions of the various selenium-dependent enzymes and their effects. The often discussed influence of selenium-dependent enzymes on the development of malignant tumors depends on numerous other factors and has not yet been fully clarified.
Typical findings are changes in the nails , scaly skin, anemia , reduced sperm quality , liver damage, growth and bone formation disorders as well as painful functional disorders and structural disorders of the muscles (myopathy). In some cases, the latter affects the patient's ability to walk. If the heart muscles are affected ( cardiomyopathy or degenerative heart muscle changes), cardiac arrhythmias and cardiac insufficiency can occur. Chronic selenium deficiency also threatens children and adolescents with diseases of bones, cartilage and joints, as well as dwarfism ( Kashin-Beck disease ). A latent undersupply cannot be diagnosed on the basis of clinical signs .
In mammals , selenium deficiency can lead to both liver necrosis and increased incidence of liver cancer . It can also affect the maturation of the sperm cells and cause infertility. In addition, the lack of glutathione peroxidase in the lens of the eye is responsible for the increased occurrence of cataracts .
Heart and skeletal muscles
The mechanism by which damage to the musculature ( skeletal and cardiac muscles ) occurs in the event of a selenium deficiency is still unclear; the muscles (in humans and animals) are characteristic paleness. Animal experiments showed significant increases in the enzyme activities of GOT and CK as signs of muscle damage with largely selenium-free feeding and sufficient administration of all other essential food components. Corresponding increases in activity are also detectable in humans.
In people with disorders of the skeletal muscles and selenium deficiency due to malabsorption or artificial nutrition , an improvement could be achieved through the administration of selenium alone. Conversely, since not all patients with these types of diet-related selenium deficiency suffer from muscular disorders, it can be assumed that other factors (e.g. viral infections) are also involved. Muscle diseases in which selenium deficiency is considered a (co-) cause are known in veterinary medicine in ruminants , pigs and turkeys . They are known as white muscle disease , nutritive muscular dystrophy , or enzootic muscular dystrophy .
A reduction in the selenium-dependent selenoprotein N is discussed as the cause of the muscle damage. Mutations in the gene coding for the selenoprotein N gene SEPN1 are associated with a number of rare congenital, usually autosomal - recessive inherited muscle diseases , some even with a cardiomyopathy been described associated. These include multicore myopathy , rigid spine muscular dystrophy type 1 (RSMD1) and desmin-related myopathy with Mallory body-like inclusions .
The selenoprotein N is found in the endoplasmic reticulum , its function is unknown. Diseases of the nervous system caused by selenium deficiency can also be the cause of muscular disorders. Selenoprotein P is also found in the muscles, and it is also assumed that it can be (partly) responsible for muscle diseases that occur when there is a selenium deficiency.
Keshan's disease
The classic heart disease caused by selenium deficiency is known as Keshan's disease . It occurs with an intake of less than 10 µg per day (selenium level in the blood serum <20 µg / l) and typically manifests itself as cardiac insufficiency caused by dilated cardiomyopathy . The cause of the cardiac arrhythmias and weakness of the heart is that parts of the heart muscles die . The fine-tissue changes in the heart are similar to those of Friedreich's cardiomyopathy .
This disease has been seen primarily in children and young women who live in areas of China where dietary selenium intake is particularly low. The name is derived from the selenium-poor district of Keshan in the Chinese province of Heilongjiang .
Damage to cell membranes due to reduced activity of the antioxidant, selenium-containing enzymes is assumed to be the cause. However, there are indications, especially from animal experiments, that there is a connection between selenium deficiency, Coxsackie virus infection, myocarditis and the development of dilated cardiomyopathy (characteristic of Keshan's disease), which could be caused by the defense mechanism weakened by selenium deficiency. Another cause known from animal experiments is a reduced function of thioredoxin reductase (TrxR2).
The substitution of selenium prevents the disease from progressing, but does not reverse existing changes. By supplementing the diet of the people living in the corresponding area with selenium, Keshan's disease was largely eradicated.
Coronary heart disease
A reduction in glutathione peroxidase activity in serum (GPX 3), which typically occurs in a selenium deficiency, is a risk factor for cardiovascular diseases . Low selenium levels in the blood are statistically related to an increased incidence of coronary heart disease ; the data on this are not uniform, so that preventive measures using drugs containing selenium are not recommended at the present time.
thyroid
The thyroid is particularly rich in selenium. It has multiple meanings in relation to the thyroid gland, its functional disorders and diseases. The joint occurrence of a selenium deficiency with iodine deficiency can intensify the development of myxedematous cretinism .
Deiodase
Deiodases are selenium-dependent enzymes whose clinically most striking function is the conversion of the thyroid hormone thyroxine (T 4 ) into the ten times more effective triiodothyronine (T 3 ). Therefore, a selenium deficiency contributes to a reduced formation of T 3 and thus to the development of hypothyroidism .
Basic medical research has shown that a gene mutation in which the selenium-dependent SE-CIS binding protein 2 (SBP2 - responsible for the translation of selenoproteins) is reduced in amount or functionality can also cause such a reduction in deiodase.
Autoantibodies
The administration of selenium inhibits the formation of autoantibodies against thyroid peroxidase . There is evidence of an increased occurrence of iodine deficiency goiter and Hashimoto's disease (autoimmune disease of the thyroid gland ) with selenium deficiency.
Oxidative stress
As part of the production of thyroid hormones in the follicular epithelial cells, large quantities of oxygen radicals and peroxides ( oxidative stress ) are created, which are intercepted by selenium-containing peroxidases under normal conditions. If there is a selenium deficiency, the concentrations of these enzymes are reduced, the cells are damaged and the incidence of papillary thyroid carcinomas increases. In such cases, children can develop atrophy of the thyroid gland (atrophic thyroiditis). It is also discussed whether additional selenium deficiency favors the increased exposure to radicals under iodine deficiency and thus the development of thyroid autonomy .
Other organ systems and body functions
growth
In addition to the influence on the thyroid hormones already described above, a lack of selenium also leads to weakness in the muscles. In animal experiments, this deficiency led to short stature and reduced food intake.
Selenium deficiency is considered to be an important cause of diseases of bones, cartilage and joints (technically known as osteoarthropathy or osteochondropathy), which in these cases often occur together with short stature . This clinical picture is known as Kashin-Beck disease and is particularly noticeable due to deformed joints of the extremities . A genetic predisposition or a viral infection ( Coxsackie virus ) are also discussed as jointly responsible for this disease .
Nervous system
There are indications that in addition to a number of muscle and nerve diseases, selenium deficiency can be associated. In animal experiments it was shown that this deficiency does not lead directly to damage, but in particular makes the brain more sensitive to nerve toxins and more frequent disorders of the blood flow . Responsible for this is the reduced activity of glutathione peroxidase-1 (GPX 1) due to a selenium deficiency. Disorders of the deiodase influence the metabolism of the thyroid hormones and thus also the development of the brain in the embryonic phase .
reproduction
Selenium-dependent enzymes play an essential role in the maturation of sperm cells . There is also evidence that sperm mobility is impaired in the event of a selenium deficiency.
A genetic impairment of the selenium-dependent enzyme glutathione peroxidase-4 (GPX 4) leads to a reduction in male fertility and a reduced quality of the sperm .
immune system
Selenium has an immunomodulating effect and there is evidence that selenium deficiency affects the immune system . An additional daily intake of 100 µg selenium led to an increase in cytokinins and an increase in T cells as part of an experimental virus infection .
From animal experiments there are indications that the cellular immune response (for example against Cryptosporidium parvum ) is weakened by selenium deficiency and that certain viruses increase in virulence , probably due to this immune deficiency . This fact can be (partly) the cause of diseases (for example of the heart).
Epidemiological studies found a connection between selenium deficiency and the increased incidence of prostate , colon , breast , ovarian and lung cancer . In animals, but not in humans, the incidence of cancer could be reduced by enriching food with selenium. A reduction in the antioxidant effect of glutathione peroxidases, inadequate processing of procarcinogens and a change in the DNA repair mechanisms are discussed as possible causes .
liver
Selenium deficiency promotes the development of necrosis of the liver . In animal experiments, a reduction in glutathione peroxidase activity could be demonstrated as a possible cause in the case of selenium deficiency. As early as the 1950s it had been established in animal experiments that the administration of selenium (then called “factor 3”) can prevent the death of liver cells when given adequate nutrition. Another indication of the protective effect of selenium is the reduction in the frequency of occurrence of a certain form of liver cancer in extremely selenium-deficient areas of China . Hepatitis B , which is very common in these regions, and the high aflatoxin content of food are seen as the cause of the development of this cancer .
blood
Selenium deficiency can be viewed as an independent risk factor for the occurrence of anemia in humans and animals. A possible cause is the reduction in glutathione peroxidase (GPX 1) in the red blood cells, because there are indications that the administration of selenium protects these blood cells from being destroyed by oxidative stress. Another possible cause known from animal experiments is assumed to be a functional impairment of the selenium-dependent thioredoxin reductase (TrxR2), which affects blood formation .
Investigation methods
The blood selenium value (selenium content in the blood plasma or related to the red blood pigment of the erythrocytes ) is suitable for recording relevant information on the selenium content of the body . A reliable reference value for the current supply situation is the determination of selenoprotein P in the blood, for the long-term supply situation of the organism the content in nails and hair.
Selenium-dependent enzymes are formed in smaller quantities when there is a selenium deficiency. This can be recognized by their measurably reduced enzyme activity, which is why the activity of glutathione peroxidase in blood plasma or erythrocytes is also suitable as indirect evidence.
The blood selenium value is around 60–80 µg / l in Germany and 100–180 µg / l in the USA . Limitations of enzyme functions (glutathione peroxidases) can be demonstrated at values below 50 µg / l . To maintain this minimum level, a daily intake of 0.67 µg selenium per kilogram of body weight is required. In order to achieve the optimal level of glutathione peroxidase activity in serum (GPX 3), however, a daily intake of around 1 µg per kilogram of body weight is required. When interpreting, it should be noted that the activity of glutathione peroxidase in plasma (GPX 3) depends on the kidney function and the selenium content of the tubular cells of the kidneys . Even with a short-term good selenium supply, it quickly reaches a normal value. Corresponding parameters from the red blood cells (lifespan about three months) show the average supply situation during the 90 days prior to the examination.
therapy
For the treatment of diseases caused by a selenium deficiency, on the one hand, the administration of sufficient selenium is necessary depending on the cause; on the other hand, symptom-related measures may also be indicated when it comes to the treatment of secondary conditions.
prevention
The goal of preventive measures is to ensure the daily intake of sufficient selenium from food. In areas where there is a lack of selenium, the enrichment of fertilizers is also suitable. For example, in Finland , the soil and drinking water of which contain little selenium, sodium selenate was added to the artificial fertilizer, which improved the supply of this essential trace element to the population. The content of selenium in milk is directly dependent on its proportion in the animal feed used. This connection was also used in Finland and also in Sweden to improve the supply of the population.
Substitution of selenium
The only reliable indications for the use of preparations containing selenium are a proven selenium deficiency and the skin disease " seborrheic dermatitis " for local use. Its use in autoimmune thyroiditis and cancer prevention is controversial (see below). Daily administration of 200 µg selenium is recommended as a simple and inexpensive supportive treatment for HIV infection , as positive effects on viral load and CD4 cells have been demonstrated. An overdose of foods or drugs containing selenium can lead to selenosis .
cancer
Taking supplements that contain selenium reduces the incidence of prostate cancer , lung cancer, and colon cancer in some patient populations . However, the intake has no influence on the incidence of skin cancer . Precanceroses in the oropharynx respond to selenium. A general recommendation for selenium supplementation for the prevention of cancer diseases cannot be given.
Autoimmune thyroiditis
It could be shown that in the vast majority of patients with Hashimoto's thyroiditis , an autoimmune disease of the thyroid gland, a daily intake of 200 µg selenium leads to a significant decrease in thyroid peroxidase antibodies (TPO-Ab) and a normalization of the sonographic echo pattern. For patients with Hashimoto's thyroiditis, higher daily selenium intake is therefore sometimes recommended (children 50 µg, adolescents 150 µg, adults 200 µg), while other sources consider the study situation to be insufficient to recommend administration of selenium. Overall, it could not be proven that the autoimmune process on which Hashimoto's thyroiditis is based comes to a standstill or that the development of an underactive thyroid (hypothyroidism) can ultimately be prevented. An effect was only detectable in patients with very high antibody levels, none at all in children and adolescents. One study reported a positive effect of selenium therapy in endocrine orbitopathy . In summary, Grünwald and Derwahl 2014 came to the conclusion that in Hashimoto's thyroiditis, an effect of selenium therapy on the immune process and the loss of function of the thyroid has not been scientifically proven. A “cautious attitude” is therefore emerging towards selenium therapy.
In pregnant women who were positive for TPO-Ab, the rates of postpartum thyroiditis and hypothyroidism could be significantly reduced by giving selenium . Pregnant women with very high TPO-Ab are therefore often recommended to take selenium as a dietary supplement. When treating cretinism as a result of combined iodine and selenium deficiency, it should be noted that selenium may only be supplemented when the iodine level has returned to normal. Otherwise the resulting increase in deiodase activity would lead to a further loss of iodine from the damaged thyroid gland.
Historical aspects
The discovery of selenium as a chemical element goes back to Jöns Jakob Berzelius in 1817. Klaus Schwarz and Calvin M. Foltz found out in 1957 that selenium is an essential trace element. They had demonstrated its protective function against a certain type of liver disease (liver necrosis). The first selenium-containing proteins were discovered in protozoa in 1973 and classified as glutathione peroxidases. In 1990 it was demonstrated that the most important deiodase (type 1) for the function of thyroid hormones depends on selenium. Up to 2006 three genes for corresponding isoenzymes could be described.
Keshan's disease was first described in 1935 . In 1964, their frequent occurrence with a muscle disease in animals (white muscle disease) was noticed. In 1965, a country doctor from Shanxi Province , whose name has been forgotten, treated the heart disease successfully in humans for the first time by administering sodium selenite. Shortly thereafter, the "Chinese Academy of Medical Sciences" confirmed the link between selenium deficiency and Keshan's disease. From 1974 to 1977, a prospective study of children in endemic counties of Sichuan Province was carried out, which documented the reduction in the frequency of occurrence and the death rate, as well as an improvement in the clinical course.
In 1979 Andre M. van Rij published a case report on the occurrence of muscular dystrophy in a patient artificially fed with low selenium , which clearly declined after the administration of selenium.
Individual evidence
- ↑ a b c d e f g h i j k Laura Vanda Papp, Jun Lu, Arne Holmgren, Kum Kum Khanna: From Selenium to Selenoproteins: Synthesis, Identity, and Their Role in Human Health . Comprehensive Invited Review. In: Antioxidants & Redox Signaling . tape 9 , no. 7 . Mary Ann Liebert, Inc., July 2007, ISSN 1523-0864 , p. 775-806 , doi : 10.1089 / ars.2007.1528 .
- ↑ a b c d e f g h i j k l m n o p q r s t u H.-K. Biesalski u. a .: Nutritional medicine: According to the nutritional medicine curriculum of the German Medical Association. Georg Thieme Verlag, 2004, ISBN 3-13-100293-X , pp. 171ff, 207, 330, books.google.de .
- ↑ a b c d e f g h i j k l m n o p q r s t J. Köhrle, L. Schomburg: selenium, selenium proteins, selenium deficiency, selenium poisoning . In: Olaf Adam, Peter Schauder, Günter Ollenschläger (eds.): Nutritional medicine: prevention and therapy . 3. Edition. Elsevier, Urban & Fischer, 2006, ISBN 978-3-437-22921-3 , III.6, pp. 149–158 ( books.google.de ).
- ↑ a b c d e f g h i j k l m Claus Leitzmann, Claudia Müller, Petra Michel, Ute Brehme, Andreas Hahn, Heinrich Laube: Nutrition in Prevention and Therapy . A textbook. 2nd Edition. Georg Thieme Verlag, 2003, ISBN 3-8304-5273-X , p. 75 ff . ( books.google.de ).
- ↑ a b c d e f g h i j Petro E. Petrides: Trace elements . In: Georg Löffler, Petro E. Petrides, Peter C. Heinrich (Eds.): Biochemistry and Pathobiochemistry . 8th edition. Springer Verlag, Berlin 2006, ISBN 3-540-32680-4 , chap. 22 , p. 676 ( books.google.de ).
- ↑ a b German Nutrition Society (www.dge.de): DA-CH reference values for nutrient intake. 2008, dge.de accessed on March 9, 2009.
- ↑ a b c d e f g Robert M. Russell (for the German edition: Hans-Joachim F. Zunft). Vitamins and trace elements - deficiency and excess. In: Manfred Dietel, Joachim Dudenhausen, Norbert Suttorp (eds.): Harrison's internal medicine. Berlin 2003, ISBN 3-936072-10-8 .
- ↑ a b c d e f g h i H. Kasper: Nutritional medicine and dietetics. Urban & Fischer-Verlag, 2004, ISBN 3-437-42011-9 , pp. 67ff., Books.google.de .
- ↑ L. Ryšavá, J. Kubačková, M. Stránský: Iodine and selenium contents in milk from nine European countries. (PDF; 223 kB) (No longer available online.) Archived from the original on December 22, 2015 ; Retrieved August 14, 2013 . Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Cornelia A. Schlieper. Selenium. In: Schlieper: Basic questions of nutrition. Publishing house Dr. Felix Büchner, 2000, ISBN 3-582-04475-0 .
- ↑ a b c A. Töpel: Chemistry and physics of milk. Behr's Verlag, 2004, ISBN 3-89947-131-8 , pp. 341–343, books.google.de .
- ↑ a b c d Heinrich Kasper: Nutritional medicine and dietetics. 11th edition. Munich 2009, ISBN 978-3-437-42012-2 .
- ↑ BR Cardoso, C. Cominetti, SM Cozzolino: Importance and management of micronutrient deficiencies in patients with Alzheimer's disease. In: Clinical Interventions in Aging. Volume 8, 2013, pp. 531-542. PMID 23696698 , PMC 3656646 (free full text), doi: 10.2147 / CIA.S27983
- ^ A b E. Burgis: Intensive course in general and special pharmacology. Urban & Fischer-Verlag, 2008, ISBN 978-3-437-42613-1 , p. 443, books.google.de
- ↑ a b c d K. Meyer-Rankes u. a .: Guide to nutritional medicine. Urban & Fischer-Verlag, 2006, ISBN 3-437-56530-3 , pp. 68-70, books.google.de .
- ↑ a b c Lothar-Andreas Hotze, Petra-Maria Schumm-Draeger: Thyroid diseases. Diagnosis and therapy. Berlin 2003, ISBN 3-88040-002-4 .
- ↑ a b c Petro E. Petrides: Trace elements . In: Georg Löffler, Petro E. Petrides (Hrsg.): Physiologische Chemie . 4th edition. Springer Verlag, Berlin 1988, ISBN 3-540-18163-6 , chap. 21 , p. 589-590 .
- ↑ The miraculous amino acid selenocysteine. In: Florian Horn u. a .: Human biochemistry. Stuttgart 2005, ISBN 3-13-130883-4 , p. 41.
- ↑ a b P. Karlson u. a .: Karlsons biochemistry and pathobiochemistry. Thieme Verlag, 2005, ISBN 3-13-357815-4 , p. 605, books.google.de/books .
- ↑ Karina Paunescu: DNA stability and thioredoxin / thioredoxin reductase in the cell nucleus. Medical dissertation , 2003, University of Würzburg, opus-bayern.de (PDF)
- ↑ KS Vaddadi et al. a .: Low blood selenium concentrations in schizophrenic patients on clozapine. In: Br J Clin Pharmacol . 2003 March; 55 (3), pp. 307-309, PMC 1884212 (free full text).
- ^ Claus Leitzmann: Vegetarianism. Munich 2007, ISBN 978-3-406-44776-1 , p. 76.
- ↑ HK Biesalski, MM Berger, P. Brätter, R. Brigelius-Flohé, P. Fürst, J. Köhrle, O. Oster, A. Shenkin, B. Viell, A. Wendel: Knowledge of selenium - results of the Hohenheim consensus meeting. In: Akt. Ernähr.-Med. 22 (1997) pp. 224-231. Quoted from Heinrich Kasper: Nutritional medicine and dietetics. 11th edition. Munich 2009, ISBN 978-3-437-42012-2 .
- ^ A b Wolfgang Kaim , Brigitte Schwederski: Bioinorganische Chemie . On the function of chemical elements in life processes. 4th edition. Vieweg + Teubner Verlag, 2005, ISBN 3-519-33505-0 , 16.8, pp. 329-335 ( books.google.de ).
- ↑ Letter to the editor on: White muscle disease in humans: myopathy caused by selenium deficiency in anorexia nervosa under long term total parenteral nutrition. In: J Neurol Neurosurg Psychiatry. 1999; 67, pp. 829-830, jnnp.bmj.com .
- ↑ a b c Julia Fischer, Astrid Bosse, Josef Pallauf: Effect of selenium deficiency on the antioxidative status and muscle damage in growing turkeys . In: Archives of Animal Nutrition . tape 62 , no. 6 . Taylor & Francis, December 2008, ISSN 0003-942X , pp. 485-497 , doi : 10.1080 / 17450390802453468 .
- ↑ a b Patrick Chariot, Olivier Bignani: Skeletal muscle disorders associated with selenium deficiency in humans . In: Muscle & Nerve . tape 27 , no. 6 , 2003, ISSN 0148-639X , p. 662–688 , doi : 10.1002 / mus.10304 .
- ^ Waldemar Hort: Dilated cardiomyopathy . In: Waldemar Hort (Ed.): Pathology of the endocardium, the coronary arteries and the myocardium . 1st edition. Springer Verlag, Berlin 2000, ISBN 3-540-63121-6 , 6.L.III.2. Selenium deficiency, S. 992 ( books.google.de [accessed April 21, 2009]).
- ↑ Klaus Bickhardt: Diet-related muscle degeneration due to vitamin E and selenium deficiency . In: Karl-Heinz Waldmann, Michael Wendt (Hrsg.): Textbook of pig diseases . Parey Verlag, Stuttgart 2004, ISBN 3-8304-4104-5 , p. 255 ff . ( limited preview in Google Book search).
- ↑ Klaus Bickhardt: Vitamin E and selenium deficiency . White Muscle Disease, Nutritive Muscular Dystrophy, Enzootic Muscular Dystrophy. In: Martin Ganter (Ed.): Textbook of sheep diseases . 4th edition. Parey Verlag, Stuttgart 2001, ISBN 3-8263-3186-9 , 2.10, pp. 139–145 ( limited preview in Google Book search).
- ↑ a b c K. Rajeev et al. a .: Selenoprotein expression is essential in endothelial cell development and cardiac muscle function. In: Neuromuscul Disord. 2007 February; 17 (2), pp. 135-142, PMC 1894657 (free full text).
- ↑ Ferreiro et al. a .: Mutations of the selenoprotein N gene, which is implicated in rigid spine muscular dystrophy, cause the classical phenotype of multiminicore disease: reassessing the nosology of early-onset myopathies. In: American Journal of Human Genetics . Volume 71, number 4, October 2002, ISSN 0002-9297 , pp. 739-749, doi: 10.1086 / 342719 , PMID 12192640 , PMC 378532 (free full text).
- ↑ Moghadaszadeh et al. a .: Mutations in SEPN1 cause congenital muscular dystrophy with spinal rigidity and restrictive respiratory syndrome. In: Nature Genetics . Volume 29, Number 1, September 2001, ISSN 1061-4036 , pp. 17-18, doi: 10.1038 / ng713 , PMID 11528383 .
- ↑ Ferreiro et al. a .: Desmin-related myopathy with Mallory body-like inclusions is caused by mutations of the selenoprotein N gene. In: Annals of neurology. Volume 55, number 5, 2004, ISSN 0364-5134 , pp. 676-686, doi: 10.1002 / ana.20077 , PMID 15122708 .
- ↑ a b U. Schweizer u. a .: The Neurobiology of Selenium: Lessons from Transgenic Mice. In: The American Society for Nutritional Sciences J. Nutr. 134, pp. 707-710, April 2004, n.nutrition.org
- ↑ a b R. D. Semba u. a .: Low Serum Selenium Is Associated with Anemia Among Older Women Living in the Community: The Women's Health and Aging Studies I and II. In: Biol Trace Elem Res. 2006 August; 112 (2), pp. 97-107, PMC 2653257 (free full text).
- ↑ a b c M. Conrad u. a .: Essential Role for Mitochondrial Thioredoxin Reductase in Hematopoiesis, Heart Development, and Heart Function In: Mol Cell Biol. 2004 November; 24 (21), pp. 9414-9423, PMC 522221 (free full text).
- ^ A supplementary review article on "Friedreich's Kardiomyopathie" with histological images: TN James: Coronary disease, cardioneuropathy, and conduction system abnormalities in the cardiomyopathy of Friedreich's ataxia. In: Br Heart J. 1987 May; 57 (5), pp. 446-457, PMC 1277199 (free full text).
- ↑ MP Burke, K. Opeskin: Fulminant heart failure due to selenium deficiency cardiomyopathy (Keshan disease). In: Medicine, science, and the law. Volume 42, Number 1, January 2002, pp. 10-13, ISSN 0025-8024 . PMID 11848134 .
- ↑ a b Selenium deficiency and viral infection. In: The Journal of Nutrition. Volume 133, Number 5 Suppl 1, May 2003, pp. 1463S-1467S, ISSN 0022-3166 . PMID 12730444 .
- ↑ TL Miller et al. a .: Nutrition in Pediatric Cardiomyopathy. In: Prog Pediatr Cardiol. 2007 November; 24 (1), pp. 59-71, PMC 2151740 (free full text).
- ↑ Gemma Flores-Mateo, Ana Navas-Acien, Roberto Pastor-Barriuso, Eliseo Guallar: Selenium and coronary heart disease: a meta-analysis. In: Am J Clin Nutr. 2006 October; 84 (4), pp. 762-773, PMC 1829306 (free full text).
- ↑ a b c d R. Gärtner: Autoimmune thyroiditis - When is thyroid hormone replacement? - When to add selenium? - Infoline thyroid ( DocCheck password required).
- ↑ K. Krohn: Oxidative stress in the thyroid glands of mice and rats with iodine and selenium deficiency. In: Universität Leipzig, Research Report 2006 - Projects , uni-leipzig.de ( Memento of the original from June 10, 2008 in the Internet Archive ) Info: The archive link has been inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. Retrieved March 11, 2009.
- ^ Andrological disorder . In: Ingrid Gerhard, Axel Feige (Hrsg.): Obstetrics integrative: Conventional and complementary therapy . 1st edition. Elsevier, Urban & Fischer, 2005, ISBN 978-3-437-56510-6 , chap. 10 , p. 402–403 ( books.google.de [accessed May 1, 2009]).
- ↑ CS Broome et al. a .: An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. In: American Journal of Clinical Nutrition. Vol. 80, No. 1, pp. 154-162, July 2004, ajcn.org .
- ↑ C. Wang et al. a .: Induced Susceptibility of Host Is Associated with an Impaired Antioxidant System Following Infection with Cryptosporidium parvum in Se-Deficient Mice. In: PLOS ONE . 2009; 4 (2), p. E4628, PMC 2644759 (free full text).
- ↑ J. Bunyan et al. a .: Protective Effect of Trace Elements other than Selenium against Dietary Necrotic Liver Degeneration. In: Nature . 181, 1801 (June 28, 1958), nature.com .
- ↑ BE Hurwitz u. a .: Suppression of Human Immunodeficiency Virus Type 1 Viral Load With Selenium Supplementation: A Randomized Controlled Trial. In: Arch Intern Med . 2007; 167, pp. 148-154, ama-assn.org .
- ^ Adriane Fugh-Berman (for the German edition: Dietrich Grönemeyer , Yvonne Kalliope Maratos): Alternative medicine / Alternative medical healing methods. In: Manfred Dietel, Joachim Dudenhausen, Norbert Suttorp (eds.): Harrison's internal medicine. Berlin 2003, ISBN 3-936072-10-8 .
- ^ Otis W. Brawley, Barnett S. Kramer (for the German edition: Steffen Hauptmann): Prevention and early detection of cancer. In: Manfred Dietel, Joachim Dudenhausen, Norbert Suttorp (eds.): Harrison's internal medicine. Berlin 2003, ISBN 3-936072-10-8 .
- ↑ a b Are there any indications for selenium administration? Pharmaceutical Information 20 (2). Innsbruck, June 2005, i-med.ac.at .
- ↑ a b c d e Frank Grünwald, Karl-Michael Derwahl: Diagnosis and therapy of thyroid diseases. Frankfurt / Berlin 2014, ISBN 978-3-86541-538-7 , pp. 63/64.
- ↑ R. Negro et al. a .: The influence of selenium supplementation on postpartum thyroid status in pregnant women with thyroid peroxidase autoantibodies. In: The Journal of Clinical Endocrinology and Metabolism . Volume 92, Number 4, April 2007, pp. 1263-1268, ISSN 0021-972X . doi: 10.1210 / jc.2006-1821 . PMID 17284630 .
- ↑ Klaus Schwarz, Calvin M. Foltz: Selenium as an integral part of factor 3 against dietary necrotic liver degeneration. In: J. Am. Chem. Soc. 1957, 79 (12), pp. 3292-3293, doi: 10.1021 / ja01569a087 .
- ^ TO Cheng: Selenium deficiency and cardiomyopathy. In: JR Soc Med. 2002 April; 95 (4), pp. 219-220, PMC 1279532 (free full text).
- ^ Andre M. van Rij, Christine D. Thomson, Joan M. McKenzie, Marion F. Robinson: Selenium deficiency in total parenteral nutrition . In: The American Journal of Clinical Nutrition . tape 32 , no. October 10 , 1979, ISSN 0002-9165 , pp. 2076-2085 ( ajcn.org [PDF]).


