Serine
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
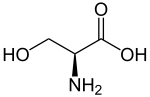
|
||||||||||||||||||||||
| Illustration of the naturally occurring L -serine | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Serine | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 3 H 7 NO 3 | |||||||||||||||||||||
| Brief description |
white, needle-shaped, sweet-tasting crystals |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 105.09 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
215-225 ° C |
|||||||||||||||||||||
| pK s value |
|
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Serine , abbreviated to Ser or S , is in the L configuration [( S ) configuration] a proteinogenic , non-essential α - amino acid .
Isomers
Serine has a stereogenic center and therefore has two enantiomers , the D -amino acid D -serine [synonym: ( R ) -serine] and its mirror image, the “natural” L -serine. The racemate DL -Serine [Synonym: ( RS ) -Serine] consists in equal parts of L -serine and D -serine. When serine is mentioned in the scientific literature without any additional addition ( prefix ), L- serine is almost always meant.
| Isomers of serine | ||
| Surname | L -Serin | D -Serin |
| other names | ( S ) -serine | ( R ) -Serine |
| Structural formula |  |
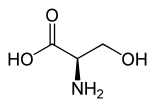
|
| CAS number | 56-45-1 | 312-84-5 |
| 302-84-1 (unspec.) | ||
| EC number | 200-274-3 | 206-229-4 |
| 206-130-6 (unspec.) | ||
| ECHA info card | 100,000,250 | 100.005.665 |
| 100.005.574 (unspec.) | ||
| PubChem | 5951 | 71077 |
| 617 (unspec.) | ||
| DrugBank | DB00133 | DB03929 |
| - (unspec.) | ||
| Wikidata | Q183290 | Q27077119 |
| Q26997410 (unspec.) | ||
L -serine racemizes (partially) more easily than other proteinogenic L -amino acids. That is why many L -serine preparations contain small amounts (0.5 to 3%) of D -serine.
Occurrence
For the first time, L- serine was isolated from silk , more precisely from the silk bast , also called sericin , which glues the fibroin threads together in the raw silk and is removed during deboning.
The enveloping bast of the silk thread, which the larva of the silk moth ( Bombyx mori ) spins into a cocoon, consists of one third of serine. Its name goes back to the Latin word sericus 'silk'.
Serine is an essential component of phosphatidylserines , a group of phosphoglycerides in the lipid bilayer of the cell membrane .
properties
At neutral pH, serine is mainly present as a zwitterion , the formation of which can be explained by the fact that the proton of the carboxy group migrates to the electron pair of the nitrogen atom of the amino group :
The zwitterion does not migrate in the electric field because it is uncharged as a whole. Strictly speaking, this is the case at the isoelectric point (at a certain pH value), at which the serine also has its lowest solubility in water. The isoelectric point of serine is 5.68.
Just like all amino acids with a ( hydrophilic ) OH group ( hydroxyl group ), serine can be phosphorylated and thus plays an important role in the activation or inactivation of enzymes . In addition, it is often located in the active center of enzymes and therefore plays an important role in biocatalysis: Examples are the serine proteinases and their inhibitors, the serpins (serine proteinase inhibitors).
Biosynthesis and degradation
For biosynthesis and degradation including structural formulas see section web links.
Serine is synthesized by oxidation and subsequent transamination starting from 3-phosphoglycerate . In the body, serine is broken down to glycine , but it can also be converted to pyruvate in a PALP- dependent, eliminating deamination by serine dehydratase .
Technical manufacturing
L -serine is produced industrially by fermentation, in an estimated amount of 100–1000 tons per year. Alternatively, keratin- containing proteins can be hydrolyzed with hydrochloric acid and neutralized with ammonia . The mixture of about 20 proteinogenic amino acids obtained in this way (one of which is L -serine) is separated due to different solubilities and by means of ion exchange chromatography . The individual fractions are purified by recrystallization.
D -Serin
In glial cells and neurons is D -serine by the enzyme serine racemase formed. D -serine acts as an endogenous co-agonist at NMDA receptors , it binds to the NR1 subunit and increases the affinity of glutamate at this receptor. There are indications that a physiological deficiency of D -serine could play a role in the process of depression .
In some plants, the serine racemase has been detected in pistils and ovules, where it plays a role in the navigation of the ingrown pollen tube. L -Serin is reconfigured to D -Serin and recognized by the pollen tube. GLR genes ( glutamate receptor- like genes) form Ca 2+ channels in the pollen tube, which are activated by D -serine, creating an oscillating Ca 2+ signal in the pollen tube tip that promotes and directs growth. Pollen tubes in which this signal path has been disrupted show deformed growth, branch out and are less fertile.
This plant signaling mechanism is interesting insofar as amino acid-mediated communication has so far been associated with the central nervous system of higher animals.
Web links
Individual evidence
- ↑ a b c d Entry on L-Serine. In: Römpp Online . Georg Thieme Verlag, accessed on May 29, 2014.
- ↑ a b Data sheet Serine (PDF) from Merck , accessed on March 14, 2010.
- ↑ a b data sheet DL-Serine, ≥98% (TLC) from Sigma-Aldrich , accessed on February 26, 2013 ( PDF ).
- ↑ Hans-Dieter Jakubke, Hans Jeschkeit: Amino acids, peptides, proteins. Verlag Chemie, Weinheim 1982, ISBN 3-527-25892-2 , p. 470.
- ↑ PM Hardy: The Protein Amino Acids. In: GC Barrett (Ed.): Chemistry and Biochemistry of the Amino Acids. Chapman and Hall, 1985, ISBN 0-412-23410-6 , p. 9.
- ↑ K. Drauz, I. Grayson, A. Kleemann, H.-P. Krimmer, W. Leuchtenberger, C. Weckbecker: Amino Acids. In: Ullmann's Encyclopedia of Industrial Chemistry . Wiley-VCH Verlag, Weinheim 2012, doi : 10.1002 / 14356007.a02_057.pub2 .
- ↑ L. Pollegioni, S. Sacchi: Metabolism of the neuromodulator D-serine . In: Cell. Mol. Life Sci. tape 67 , no. 14 , 2010, p. 2387-2404 , doi : 10.1007 / s00018-010-0307-9 , PMID 20195697 .
- ↑ O. Malkesman, DR Austin, T. Tragon et al: Acute D-serine treatment produces antidepressant-like effects in rodents . In: Int. J. Neuropsychopharmacol. tape 15 , no. 8 , 2012, p. 1135-1148 , doi : 10.1017 / S1461145711001386 , PMID 21906419 .
- ↑ Erwan Michard, Pedro T. Lima, Filipe Borges, Ana Catarina Silva, Maria Teresa Portes: Glutamate Receptor – Like Genes Form Ca2 + Channels in Pollen Tubes and Are Regulated by Pistil d-Serine . In: Science . tape 332 , no. 6028 , April 22, 2011, p. 434–437 , doi : 10.1126 / science.1201101 , PMID 21415319 ( sciencemag.org [accessed November 11, 2016]).

