Terciopelo lance viper
| Terciopelo lance viper | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|

Bothrops asper (young animal) |
||||||||||||
| Systematics | ||||||||||||
|
||||||||||||
| Scientific name | ||||||||||||
| Bothrops asper | ||||||||||||
| ( Garman , 1844) |
The terciopelo lance viper ( Bothrops asper ) is a species of snake that is widespread in Central America and northwestern South America . It belongs to the subfamily of pit vipers . Other names for the species that are occasionally used in the German-speaking area are American lance viper , Rauschuppige lance viper , Fer de lance or Barba amarilla . The species inhabits tropical deciduous and rainforests. There it lives mainly on the ground, but also climbs at least a few meters high on trees or bushes. Young bothrops asper feed mainly on small amphibians , reptiles and invertebrates , adult animals mainly on small mammals .
The Terciopelo lance viper becomes very large with a body length of over two meters, is easily excitable, moves very quickly and is extremely poisonous. It is responsible for the majority of snake bite poisoning within its area and for numerous deaths every year. Many patients who survive the bite remain handicapped for life due to severe tissue damage and limb losses.
description
Bothrops asper is the largest species of the genus, but is relatively slim compared to other members of the genus. Sexually mature individuals are usually between 1.2 and 1.8 m long, the largest specimens reach a body length of 2.5 m. Females are already larger than males at birth and later also attain significantly larger body dimensions.
Scaling
The species shows a single loreal and 5–11, mostly 6–9, keeled supraocularia on the head sides . The number of supralabials is 7–9 (usually 7), the number of infralabials 8–12, usually 10 or 11. The number of ventral scales ( ventral shields ) varies between 161 and 240, the number of subcaudalia between 46 and 81 and the number of the dorsal rows of scales in the middle of the body between 23 and 33, mostly 25-29.
coloring
Coloring and drawing of the snake are very variable. The basic color of the top can be red-brown, brown, olive-green, gray-brown, pink or almost black. The top of the head is usually not drawn. A dark stripe (postocular stripe) extends from the eye to the corner of the mouth and can enclose the back one or two shields of the upper lip ( supralabials ). The upper side shows on both sides of the back 18 to 28 light-edged, dark brown to almost black triangles, the broad base of which points towards the belly. Often there are large, dark points in the ventral extension of both legs of the triangle. The triangles can meet with their tips in the middle of the back, so that the back shows a very conspicuous X-drawing, or they can be offset from one another. Towards the tail, the drawing becomes increasingly narrow, the tail itself is usually a single color, dark brown or black. The belly side is usually yellow, rarely cream-colored or white-gray and shows irregular, dark spots that become denser towards the tail.
Male young animals have a yellow tail end, in female young animals it is a solid brown. In males, the yellow tail color begins to fade at a body length of about 100 cm, it disappears until sexual maturity.
distribution and habitat
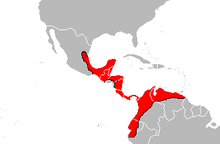
The distribution area of the Terciopelo lance viper extends from northeast Mexico to the south over all of Central America. It also includes the coastal northwest of South America from Ecuador in the south via Colombia to the eastern border of Venezuela . The southern limit of distribution in Colombia and Venezuela is controversial and needs to be further investigated, especially the delimitation to the southeast adjoining, very similar species Bothrops atrox is unclear in many areas. However, where both species occur parapatric or sympatric , there is apparently an ecological separation.

B. asper can be found in Mexico and Central America mainly in the lowlands, the highest sites there were at 1300 m above sea level. In South America it also inhabits significantly higher areas, in Colombia it was found up to an altitude of 2640 m, in Venezuela at an altitude of 2500 m. The habitat of the Terciopelo lance viper is primarily tropical rainforests , cloud forests and tropical evergreen forests; it is also common in the fringes of savannahs . In addition, drier landscapes such as tropical deciduous forests, thorn forests or pine savannas are also populated; however, it is much rarer in such habitats and can usually only be found near water. The species also colonizes secondary forests and probably also likes to visit settlements in search of food.
Systematics
No subspecies of the Terciopelo lance viper are described. The systematic differentiation of B. asper from B. atrox is sometimes controversial, and the combination of the two species into a superspecies has also been proposed. However, according to recent molecular genetic work, the separation of the two species is well founded. In the most comprehensive molecular genetic work to date, which considered 28 species or forms of the genus, the Terciopelo lance viper is the sister species of a clearly defined group of seven Bothrops species, which also includes B. atrox .
behavior
The snake's activity is evidently subject to seasonal fluctuations. At the northern limit of its distribution, the species is significantly less active in winter; in the south of the area, activity is significantly higher during the rainy season than in the dry season. Like all representatives of the genus, Bothrops asper is predominantly nocturnal. The day is regularly spent sunbathing in clearings or on river banks, but often in the shade of higher vegetation. Bothrops asper is predominantly ground-living, but is often found a few meters above the ground in low vegetation or on tree trunks.
nutrition
Little is known about the nocturnal hunting methods of adult Terciopelo lance vipers. Systematic studies on nutrition are also hardly available to date. They mainly feed on small mammals , as well as birds , reptiles and amphibians. House rats , opossum rats ( Philander sp., Didelphis marsupialis , Caluromys derbianus ) and pocket mice ( Heteromys sp.) Have often been identified as prey. Individual records concerned a Brazilian cottontail ( Sylvilagus brasiliensis ), a cotton rat ( Sigmodon peruanus ), the gecko Gonatodes fuscus , a juvenile Bothrops asper , a representative of the real frogs ( Rana forreri ) and an undetermined bird.
Young animals use their tail end as bait. When amphibians and reptiles come near, the snake aligns its head in their direction and exposes the tail end. The basal part of the tail is held vertically, the end of the tail is then rotated or pivoted in a horizontal position. Frogs (and presumably reptiles) are bitten and held until they stop moving. Young animals apparently mainly eat amphibians and reptiles, as well as invertebrates . Evidence were u. a. Anoles and skinks , frogs (e.g. Antilles whistling frogs Eleutherodactylus sp.), Large centipedes and grasshoppers .
In Ecuador , the stomach contents of 14 individuals of different ages were examined. Six of these contained remains of mice (Muridae), three remains of the whistling frog Eleutherodactylus achatinus and nine remains of insects. One individual had the remains of a lizard in their stomach, another had the remains of a Thryothorus nigricapillus belonging to the wrens family, and a large centipede.
Reproduction
Like all species of the genus, the Terciopelo lance viper is viviparous . Within the genus Bothrops there is a clear connection between body size and number of young animals; B. asper is the most fertile member of the genus.
Within the large distribution area, the phenology and the number of young animals often vary considerably, even on a small scale. In Costa Rica , the occurrences are largely limited to the coastal lowlands in the north and south of the country, the mountain range in the center of the country is almost unpopulated. On the Atlantic (northern) side, mating takes place in March, the young are born from September to October, the number of young snakes was between 14 and 86, with an average of 41. On the Pacific side, mating takes place from September to November, the birth of the pups from April to June and the number of pups was only 5 to 40, on average 19. In both populations, a clear correlation between the size of the females and the number of pups was determined, more than 60 pups were only found in females of more than 1 , 8 m length found. The young animals of both populations showed hardly any size differences, the body length was between 27 and 36.5 cm, the weight between 6.1 and 20.2 g. The parameters of the populations from other regions are within the values given here for Costa Rica.
Age
Information on the average and maximum ages of individuals living in the wild is unknown; the maximum age in captivity was over 20 years.
Behavior towards people
Bothrops asper is considered to be very excitable. If it is deliberately disturbed, it moves very quickly, changes direction abruptly and tries to bite. If it is illuminated with a flashlight in the dark, it looks for cover, but then often returns to the point of disturbance. During the day, when someone approaches, she does not flee, but trusts in her excellent camouflage and remains motionless. It only bites very quickly when it falls below a certain distance or when it is touched. The majority of those bitten therefore only notice the snake at the moment of biting. Before the bite, the snake stands up, so many people are bitten above the knee.
Toxic effect on humans
The toxin mixtures of pit vipers are by far komplexesten natural poisons. They contain a mixture of enzymes , low molecular weight polypeptides , metal ions and other components whose function has so far hardly been understood. The effects of these poisons are correspondingly diverse. Bothrops asper's venom causes a number of symptoms, a distinction being made between local and whole-body ( systemic ) symptoms. Particularly problematic with this very large species is the large amount of poison administered in one bite and the high level of toxicity. The average amount of poison per bite is 458 mg (dry weight), a maximum of 1530 mg, the LD 50 value in mice is 3.7 mg per kg of body weight.
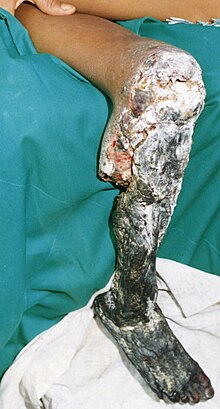
Local effects
The poison contains tissue- destroying enzymes , especially phospholipase A 2 and highly protein-degrading metalloproteinases . Typical local symptoms are above all severe pain, redness and swelling that quickly spreads to the entire bitten limb and the adjacent trunk, as well as small or large blisters that contain clear or blood- serous fluid. Severe necrosis often occurs , particularly of the muscle tissue. If treatment is not initiated or is initiated too late, the affected limbs often have to be amputated because of the necrosis; even with timely treatment, affected areas often have to be surgically restored. Further permanent damage is functional impairment or loss due to muscle wasting ( atrophy ), permanent muscle shortening and paralysis of peripheral nerves.
Systemic effects
The poison has a hemolytic effect and, due to metalloproteinases, has a hemorrhagic effect (destroys blood vessels). It caused by thrombin-like enzymes ( TLEs ) a change in the blood coagulation precursor fibrinogen and thereby a pathological activation of clotting . This leads to the rapid consumption of the coagulation factors via further steps and therefore has an anticoagulant effect . The syndrome is known as Disseminated Intravascular Coagulopathy (DIC). The patients bleed from the bite site, from unhealed scars, mosquito bites and oral mucous membranes and internal bleeding occurs. The poison is apparently also directly toxic to the kidneys. In pregnant women, the bites often lead to spontaneous abortions . Additional complications arise from infections from the bacterial fauna contained in the snake's mucous membranes. The most common causes of death are acute kidney failure, cerebral haemorrhage and blood poisoning.
Epidemiology
The combination of high levels of poison, high toxicity, low willingness to flee, comparatively high level of aggressiveness, frequent residence in human settlements and relatively wide distribution makes B. asper by far the most medically relevant snake in its area. It is responsible for the majority of snake bite poisoning and almost all deaths within its area.
In a study on the epidemiology of snake bites in children and adolescents in Costa Rica between 1985 and 1995, 65% of the poisonings were due to Bothrops asper , 3 of the 79 children bitten died. Across Costa Rica, an average of 504 people were bitten by poisonous snakes per year in the period 1990–2000. The number of deaths ranged from 0 to 7 per year. The majority of bites and almost all deaths were attributed to B. asper . Overall, it was possible there to reduce the mortality among the bite victims considerably, in 1947 it was still 7%, in the 1990s it was almost 0%.
In two provinces in northwest Colombia , an average of 669 people were bitten by snakes each year in the 1990s. 50 to 70% of the bites were caused by B. asper , the mortality was 5%, and a further 6% suffered permanent damage. Of 244 snake bite victims in these two provinces between March 1989 and February 1990, 44.5% were B. asper . 12 patients (4.9%) died, a further 13 (5.3%) were permanently disabled. Similar figures are available from other countries within the distribution area of B. asper .
Most B. asper bites occur in rural areas, affecting young people who work in agriculture in particular. In Costa Rica, 46.2% of all those bitten between 1990 and 2000 were engaged in agricultural work, a further 20% were housewives or other work in houses, and for the remainder their employment was unknown.
Other groups of people are rarely affected. Of 10 field biologists who were poisoned by snake bites in Central America between 1980 and 1991, all had been bitten by B. asper . All survived the bite, but in one case a lower leg had to be amputated, in a second case destroyed tissue had to be surgically replaced and in a third case psychological trauma prevented further work as a field biologist. However, the authors of the study put the risk of a bite into perspective, three bites occurred in 4 projects with a total of more than 1.5 million "field hours"; the risk was therefore one bite for around 500,000 hours spent in the field.
swell
Individual evidence
- ↑ Jonathan A. Campbell and William W. Lamar: The taxonomic status of miscellaneous Neotropical viperids, with the description of a new genus . Occasional papers of the Museum, Texas Tech University 155, 1992: pp. 1-33. quoted In: A. Campbell, William W. Lamar: The Venomous Reptiles of the Western Hemisphere. Comstock; Ithaca, London. 2004: p. 376
- ↑ Ronald L. Gutberlet and Michael B. Harvey: The Evolution of New World Venomous Snakes . In: Jonathan A. Campbell, William W. Lamar: The Venomous Reptiles of the Western Hemisphere. Comstock; Ithaca, London. 2004. ISBN 0-8014-4141-2 : pp. 676 and 679
- ^ W. Wüster, MG Salomão, JA Quijada-Mascareñas, RS Thorpe and BBBS P: Origin and evolution of the South American pitocket fauna: evidence from mitochondrial DNA sequence analysis. In: GW Schuett, M. Höggren, ME Douglas & HW Greene (eds): Biology of the Vipers. Eagle Mountain Publishing, Eagle Mountain, Utah, 2002: pp. 111-128.
- ↑ C. Boada, D. Salazar-V., A. Freire Lascano, U. Kuch: The diet of Bothrops asper (GARMAN, 1884) in the Pacific lowlands of Ecuador. In: HERPETOZOA. 18 (1/2) Vienna, 2005.
- ↑ ML Avila-Agüero, K. Valverde, J. Gutiérrez, MM París and I. Faingezicht: Venomous snakebites in children and adolescents: a 12-year retrospective review . Journal of Venomous Animals and Toxins 7; 2001: pp. 69-84 doi: 10.1590 / S0104-79302001000100006
- ↑ Mahmood Sasa and Silvia Vazquez: Snakebite envenomation in Costa Rica: a revision of incidence in the decade 1990-2000 . Toxicon 41, 2003: pp. 19-22 doi: 10.1016 / S0041-0101 (02) 00172-1
- ↑ R. Otero, GS Tobón, L. Fernando Gómez, R. Osorio, R. Valderrama, D. Hoyos, JE Urreta, S. Molina and JJ Arboleda: Accidente ofídico en Antioquia y Chocó. Aspectos clínicos y epidemiológicos (March 1989-February 1990). Acta Médica Colombiana 17: pp. 229-249. quoted in David A. Warrell: Snakebites in Central and South America: Epidemiology, Clinical Features, and Clinical Management : p. 737
- ↑ Mahmood Sasa and Silvia Vazquez: Snakebite envenomation in Costa Rica: a revision of incidence in the decade 1990-2000 . Toxicon 41, 2003: pp. 19-22
- ↑ David L. Hardy Sr .: Bothrops asper (Viperidae) Snakebite and Field Researchers in Middle America . Biotropica 26 (2) 1994: pp. 198-207
literature
- David A. Warrell: Snakebites in Central and South America: Epidemiology, Clinical Features, and Clinical Management . In: Jonathan A. Campbell, William W. Lamar: The Venomous Reptiles of the Western Hemisphere. Comstock; Ithaca, London. 2004. ISBN 0-8014-4141-2 : pp. 709-761.
- Jonathan A. Campbell, William W. Lamar: The Venomous Reptiles of the Western Hemisphere. Comstock; Ithaca, London. 2004. ISBN 0-8014-4141-2
Related Links
- Detailed description of first aid and other emergency measures in the event of a bite as well as rules of conduct to avoid bites online ( Memento from December 12, 2011 in the Internet Archive ) (accessed on November 30, 2013, English)