Tricosanoic acid
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
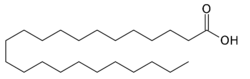
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Tricosanoic acid | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 23 H 46 O 2 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 354.61 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
0.875 g cm −3 |
|||||||||||||||||||||
| Melting point |
77-79 ° C |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Tricosanoic acid is an organic compound from the group of alkanoic acids . It is a long-chain, saturated fatty acid and its salts and esters are called tricosanoates .
Occurrence
Tricosanoic acid occurs naturally in the leaves of Cecropia adenopus and in fennel . It is also found in small amounts in lipids from various other plants.
Extraction and presentation
Tricosanoic acid can be obtained by reductive desulphurization of the corresponding 5-alkyl-2-thiophenecarboxylic acid.
Individual evidence
- ↑ a b c d Data sheet Tricosanoic acid, ≥99% (capillary GC) from Sigma-Aldrich , accessed on September 18, 2015 ( PDF ).
- ^ Carl L. Yaws: Thermophysical Properties of Chemicals and Hydrocarbons . William Andrew, 2014, ISBN 978-0-323-29060-9 ( books.google.com ).
- ↑ Wolfgang Blaschek, Rudolf Hänsel, Konstantin Keller, Jürgen Reichling, Horst Rimpler, Georg Schneider: Hager's Handbook of Pharmaceutical Practice: Volume 2: Drugs AK . Springer-Verlag, 2013, ISBN 978-3-642-58928-7 ( books.google.com ).
- ↑ TK Lim: Edible Medicinal And Non-Medicinal Plants. Volume 5: Fruits , Springer, 2013, ISBN 978-94-007-5652-6 , p. 43.
- ↑ Tricosanoic acid ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice. at PlantFA Database, accessed November 3, 2017.
- ↑ Salo Gronowitz: The Chemistry of Heterocyclic Compounds, Thiophene and Its Derivatives . John Wiley & Sons, 2009, ISBN 978-0-470-18875-0 ( books.google.com ).
