Tridecanoic acid
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
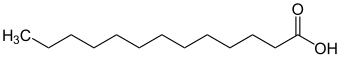
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Tridecanoic acid | |||||||||||||||||||||
| other names |
n -Tridecanoic acid |
|||||||||||||||||||||
| Molecular formula | C 13 H 26 O 2 | |||||||||||||||||||||
| Brief description |
white solid with a waxy woody odor |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 214.34 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
0.8458 g cm −3 (80 ° C) |
|||||||||||||||||||||
| Melting point |
41-42 ° C |
|||||||||||||||||||||
| boiling point |
312-313 ° C |
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| Refractive index |
1.4286 (60 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||||||||
The tridecanoic acid is a saturated long-chain fatty acid having an odd carbon number and belongs to the substance group of alkanoic acids . Their salts and esters are called tridecanoates .
Occurrence
Like most long-chain fatty acids with an odd number of carbon atoms, tridecanoic acid occurs rarely and in low concentrations in nature.
For example, it was found with proportions of 0.24% to 0.64% of the total fatty acids in some freshwater species of cyanobacteria . Also, in some vegetable oils to low levels found at tridecanoic, so to 0.07% in the leaf oil of Cuban rue ( Ruta graveolens ) and 0.3% in the Cuban star fruit oil ( Averrhoa carambola ) u. a.
It is the main fatty acid with a share of almost 90% of the fatty acids in the seeds of the Australian plant Stackhousia tryonii .
Extraction and presentation
Tridecanoic acid can be made by oxidizing 1-tetradecene with potassium permanganate .
use
Tridecanoic acid is used in concentrations of up to 8% in perfume concentrates . Because of its rarity and its low occurrence in biological material, it is also often used as an internal standard in the gas chromatographic analysis of fatty acids .
Individual evidence
- ↑ a b c d data sheet of The Good Scents Company ; June 10, 2008 .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 96th edition. (Internet Version :), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-524.
- ↑ a b c Data sheet Tridecanoic acid from Sigma-Aldrich , accessed on June 26, 2014 ( PDF ).
- ↑ SH Yalkowsky, RM Dannenfelser: Aquasol database of wässrige solubility . Version 5 .; College of Pharmacy, University of Arizona - Tucson, AZ. PC version. 1992.
- ↑ a b c David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-496.
- ↑ T. Rezanka, I. Dor, A. Prell, VM Dembitsky: Fatty acid composition of six wild freshwater cyanobacterial species. In: Folia Microbiol. 48 (1), 2003, pp. 71-75, doi : 10.1007 / BF02931279 .
- ↑ Tridecanoic acid at PlantFA Database, accessed November 7, 2017.
- ↑ Naveen P. Bhatia, Ani E. Nkang, Kerry B. Walsh, Alan JM Baker, Nanjappa Ashwath, David J. Midmore: Successful Seed Germination of the Nickel Hyperaccumulator Stackhousia tryonii. In: Annals of Botany . 96 (1), 2005, pp. 159-163, doi : 10.1093 / aob / mci151 .
- ↑ Donald G. Lee, Shannon E. Lamb, and Victor S. Chang: Carboxylic acids from the oxidation of terminal alkenes by permanganate: Nonadecanoic acid In: Organic Syntheses . 60, 1981, p. 11, doi : 10.15227 / orgsyn.060.0011 ; Coll. Vol. 7, 1990, p. 397 ( PDF ).
- ↑ Antonio Gonzalez Casado; Enrique J. Alonso Hernandez; Pedro Espinosa; Jose Luis Vílchez: Determination of total fatty acids (C8-C22) in sludges by gas chromatography-mass spectrometry. In: Journal of Chromatography A . 826 (1), 1998, pp. 49-56, doi : 10.1016 / S0021-9673 (98) 00725-0 .
