Trypanosoma brucei
| Trypanosoma brucei | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
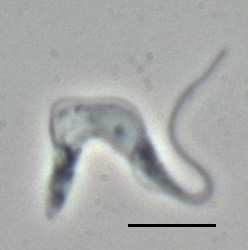
Trypanosoma brucei brucei phase contrast image of a trypomastigote; Bar 10 µm |
||||||||||||
| Systematics | ||||||||||||
|
||||||||||||
| Scientific name | ||||||||||||
| Trypanosoma brucei | ||||||||||||
| Plimmer & Bradford , 1899 |
Trypanosoma brucei is a type of single-celled parasite known as pathogens of African sleeping sickness medical human meaning. In endemic areas in Africa, the parasite circulates between blood-sucking tsetse flies and various species of mammals, including domestic animals. A subspecies of Trypanosoma brucei is a causative agent of the animal disease Nagana , which is a major problem for livestock in Africa. The parasites have an extremely complex life cycle with different life forms in insects and mammals. Trypanosoma brucei is also characterized by molecular peculiarities in the regulation of gene expression and in avoiding the immune response of the mammalian host.
Discovery and Description
history
African trypanosomes were first observed in the blood of cattle infected with Nagana in 1895 by the doctor David Bruce and identified as the cause of the disease. Bruce was also able to show the transmission by tsetse flies. The pathogen was named Trypanosoma brucei (synonym: Trypanosoma hominis ) in honor of Bruce . A few years later, trypanosomes were identified in people suffering from West African sleeping sickness ; the pathogen was named Trypanosoma gambiense after the West African river Gambia ; there the parasite was identified for the first time. Trypanosomes were also found in patients suffering from East African sleeping sickness , which were named Trypanosoma rhodesiense after the British colony of Northern Rhodesia , where it was first identified . The three originally identified species were combined into one species in 1972 under the name Trypanosoma brucei , which in turn was divided into three subspecies, Trypanosoma brucei brucei , Trypanosoma brucei gambiense and Trypanosoma brucei rhodesiense .
morphology
The unicellular has a single flagellum , which runs on the cell surface under an undulating membrane to the front end of the cell and there will in most forms of the parasite to a freely oscillating scourge. The cells also have a small kinetoplast, a collection of DNA within a large mitochondrion . The parasite occurs in several pleomorphic cell forms:
- The trypomastigote form, in which the base of the flagellum is posterior to the cell nucleus , occurs in the blood of mammals either as a slender, up to 40 µm long form ( slender ) with a long, free flagellum and subterminal kinetoplast or as a short, squat, up to 25 µm long form ( stumpy ) without free flagella end and a kinetoplast near the posterior end; in between there are also intermediate forms. There is also a procyclical and a metacyclical form; both occur in tsetse flies. The metacyclical form has no free scourge.
- The epimastigote form, in which the flagella base and the kinetoplast lie anterior to the cell nucleus, is otherwise similar to the trypomastigote and also only occurs in flies.
The three subspecies cannot be distinguished microscopically. The species Trypanosoma evansi , the causative agent of the Surra , and Trypanosoma equiperdum , the causative agent of the malignancy , are microscopically indistinguishable from Trypanosoma brucei .
Systematics
Within the genus Trypanosoma , Trypanosoma brucei is classified together with Trypanosoma evansi and Trypanosoma equiperdum in the subgenus Trypanozoon . Below the species, three subspecies and other groups without taxonomic rank are distinguished:
-
Trypanosoma brucei
-
Trypanosoma brucei brucei (formerly Trypanosoma brucei )
- "Brucei"
- "Kiboko"
- "Sindo"
-
Trypanosoma brucei gambiense (formerly Trypanosoma gambiense )
- "Group 1"
- "Group 2"
-
Trypanosoma brucei rhodesiense (formerly Trypanosoma rhodesiense )
- "Northern"
- "Southern"
-
Trypanosoma brucei brucei (formerly Trypanosoma brucei )
Various molecular studies have shown that the differences between the subspecies of Trypanosoma brucei , but also between all other representatives of the subgenus Trypanozoon , are very small. Trypanosoma brucei brucei and Trypanosoma brucei rhodesiense are essentially different by a single gene for a Serum Resistance Associated (SRA) factor. If the gene for this factor is transferred to Trypanosoma brucei brucei , this parasite becomes infectious for humans. The SRA protein belongs to the family of variable surface glycoproteins (VSG). It is believed that the SRA gene from Trypanosoma brucei rhodesiense was generated by partial deletion in a VSG gene from Trypanosoma brucei brucei and then spread between different Trypanosoma brucei strains by recombination.
Trypanosoma evansi and Trypanosoma equiperdum differ from Trypanosoma brucei practically only by completely ( evansi ) or partial ( equiperdum ) absence of kDNA- Maxi Circles , a mitochondrial DNA . The consequence of this difference is that both species cannot reproduce in tsetse flies; they are transmitted purely mechanically. The small molecular differences within the subgenus Trypanozoon would hardly justify any further taxonomic differentiation. however, these differences lead to clearly different host specificities and different disease courses, which means that the distinction is of great practical importance.
Distribution and host animals
All three subspecies of Trypanosoma brucei come almost exclusively in sub-Saharan Africa in the area of distribution of the tsetse fly, the so-called Tsetsegürtel before. The distribution areas of Trypanosoma brucei gambiense and Trypanosoma brucei rhodesiense are separated by the East African rift valley; only in Uganda are both subspecies observed, but in separate distribution areas. Trypanosoma brucei brucei can be found in the entire Tsetsa area.
For Trypanosoma brucei gambiense , humans are the main reservoir . The pathogen was also isolated from pigs and sheep, and in individual cases also from monkeys, but its epidemiological significance as a reservoir is controversial. Monkeys can be infected experimentally without becoming seriously ill. These parasites are transmitted by flies of the Glossina palpalis group, which mainly live near rivers. The reason why humans are susceptible to infection with this pathogen lies in the pathogen's resistance to two human proteins, the trypanolytic factors (TLF-1 and TLF-2).
For Trypanosoma brucei rhodesiense , beef is the main reservoir; In epidemic areas, up to 20 percent of cattle can be acutely or chronically infected with parasites that are pathogenic to humans. In addition to cattle, domestic animals such as pigs and goats are other reservoirs. Parasites were also often isolated from various wild animals, for example the bushbuck and other antelopes . Epidemiologically, wild animals no longer play a dominant role due to the declining areas of distribution of the animals, but pets and, as a result, humans can be infected from wild animal reservoirs. Experimental infection in monkeys is possible and in most cases fatal. It is transmitted by flies of the Glossina morsitans group, which mainly live in the savannah .
Trypanosoma brucei brucei can infect many domestic mammals, camels, and numerous wild animals, including various antelopes and some carnivores, but not humans. The course of the infection is very different; some West African domestic cattle breeds show hardly any signs of disease, while East African zebu cattle are particularly sensitive. Infections in horses, camels, dogs and cats are usually severe and often fatal. The apolipoprotein LI (APOL1), a protein that was first found in humans and gorillas, is responsible for human resistance to Trypanosoma brucei brucei and some other trypanosomes . When trypanosomes ingest APOL1 through endocytosis , APOL1 forms pores in the membrane of the lysosomes , which lead to lysis of the parasite cells. This selection advantage is bought at the cost of an increased risk of developing certain kidney diseases ( nephrosclerosis , focal segmental glomerulosclerosis ), the frequency of which is correspondingly higher in people of black African descent. The serum resistance of humans due to APOL1 is overcome by Trypanosoma brucei rhodesiense by means of the Serum Resistance Associated (SRA) factor, in that the SRA protein binds to the apolipoprotein and neutralizes its trypanolytic effect. Trypanosoma brucei gambiense does not have an SRA; the mechanism of serum resistance in this subspecies is unknown.
Trypanosomes can in principle also be transferred mechanically, for example using medical equipment. Transmission from the mother to the embryo does occur, but does not play a role epidemiologically; transmission by transfusion appears possible, but is not documented. A purely mechanical transmission through his calves has been proven experimentally, but the epidemiological significance is unclear. There are, however, indications of cases of Nagana with Trypanosome brucei. outside of the tsetse distribution area.
Life cycle
Trypanosoma brucei reproduces exclusively extracellularly . After the bite of an infected tsetse fly, metacyclic trypomastigote cells enter the tissue of the mammalian host with the saliva, where they transform into slender trypomastigotes in the cell space and multiply through longitudinal division. From there, the cells enter the lymphatic system and bloodstream, where they continue to multiply. In the further course of the infection, the slender trypomastigotes sometimes transform into short, compact trypomastigotes; these no longer multiply, but are infectious for the flies. Starting from the parasites in the bloodstream, the central nervous system can also be attacked, which then leads to the symptoms of African sleeping sickness .
When a tsetse fly stings an infected mammal, it can ingest parasite cells in the blood. By such a blood meal taken squat Trypomastigote transform into the fly in the midgut to procyclical trypomastigotes. The cell surface of the parasite changes massively: the shell of variable surface glycoproteins is replaced by a surface coat of procycline protein; Simultaneously the energy metabolism of a in a particular organelle , the Glycosom running glycolytic to an oxidative converted ATP generation in mitochondria.
The procyclical trypomastigotes reproduce in the bowel of the fly, transform into epimastigotes and migrate to the salivary gland , where they continue to reproduce. Epimastigotes are the only form of parasite that can exchange genetic information through recombination ; Multiple infections with different trypanosomes are often observed in tsetse flies, so that a regular genetic exchange seems possible. From the epimastigotes, new metacyclic trypomastigote cells emerge in the salivary gland, which again carry a shell made of variable surface glycoproteins. These cells can infect a new mammalian host via the fly's saliva after a bite. The reproduction cycle in the fly lasts 20 to 40 days; the flies remain infectious for their entire lifespan.
Molecular Properties and Therapy
The complete genome - the DNA sequence of Trypanosoma brucei brucei was published in the year of 2005. The 26 mega base pair genome in 11 large chromosomes contains around 9,000 different genes, half of which have no known function. In addition to the large chromosomes, there are also intermediate chromosomes and mini-chromosomes. The genome is largely transcribed into polycistronic mRNAs ; this is very unusual in eukaryotes . Also, the trans-splicing in the exons of different transcripts are assembled, is a particular feature of the trypanosomes that is rarely seen in other eukaryotes.
The Trypanosoma brucei genome contains more than 1000 genes for variable surface glycoproteins (VSG). By frequently changing the gene expression of the various VSG genes, the parasite can periodically change its cell surface and thus avoid the specific immune response of the mammalian host's immune system . However, most of these VSG genes appear to be pseudogenes . In addition to the antigen variation , hydrodynamic effects, through which the trypanosomes can strip antibodies of the host from their shell, also play a role in evading the activity of the immune system.
Four drugs against Trypanosoma brucei are available for human medicine and their use should only be carried out after consultation with a center experienced in therapy. All four have severe side effects ; Resistance is also increasingly observed. Of the substances currently used, only eflornithine (used mainly in Trypanosoma brucei gambiense in the second, encephalitic, stage) has a specific mechanism of action as an inhibitor of the enzyme ornithine decarboxylase. Pentamidine (especially in Trypanosoma brucei gambiense in the first, hemo-lymphatic, stage), suramin (especially in Trypanosoma brucei rhodesiense in the first stage) and (as an alternative to eflornithine and in Trypanosoma brucei rhodesiense in the second stage) melarsoprol have a relatively unspecific effect; the effectiveness is attributed to a selective uptake of the drugs by the trypanosomes. The same applies to the substances diminazen and ethidium bromide used in veterinary medicine . The previously used tryparsamide is no longer used due to resistance and severe side effects since the early 1970s.
There is therefore an urgent need for new antiprotozoal agents which are effective against Trypanosoma brucei . New drugs against trypanosomes have not been developed for decades, although there are a number of specific biological processes and structures with the kinetoplasts, the glycosomes and the trans-splicing. The mechanism of purine uptake by the trypanosomes is currently regarded as a promising target for pharmaceutical research ; Trypanosoma brucei is unable to synthesize purines itself and has to absorb them from the host's body fluid.
See also
Individual evidence
- ↑ August Stich, Dietmar Steverding: The return of an epidemic: Trypanosomes. In: Biology in Our Time . Volume 32, No. 5, 2002, pp. 294-302. doi : 10.1002 / 1521-415X (200209) 32: 5 <294 :: AID-BIUZ294> 3.0.CO; 2-G
- ↑ D. Steverding: The history of African trypanosomiasis. In: Parasite Vectors. 1 (1), Feb 12, 2008, p. 3. PMID 18275594
- ^ A b W. Gibson: Resolution of the species problem in African trypanosomes. In: Int J Parasitol. 37 (8-9), Jul 2007, pp. 829-838. PMID 17451719 .
- ^ WC Gibson: The SRA gene: the key to understanding the nature of Trypanosoma brucei rhodesiense. In: Parasitology. 131 (Pt 2), Aug 2005, pp. 143-150. PMID 16145931
- ↑ J. Pépin, HA Méda: The epidemiology and control of human African trypanosomiasis. In: Adv Parasitol. 49, 2001, pp. 71-132. PMID 11461032
- ↑ Pierrick Uzureau, Sophie Uzureau et al .: Mechanism of Trypanosoma brucei gambiense resistance to human serum. In: Nature . 501, 2013, pp. 430-434, doi: 10.1038 / nature12516 .
- ↑ EM Fèvre, K. Picozzi, J. Jannin, SC Welburn, I. Maudlin: Human African trypanosomiasis: Epidemiology and control. In: Adv Parasitol. 61, 2006, pp. 167-221. PMID 16735165
- ^ CJ Maré: African animal trypanosomiasis. In: United States Animal Health Association (Ed.): Foreign Animal Diseases. St. Joseph, MO, 1998.
- ↑ Cassandra Will Yard: Putting sleeping sickness to bed. In: nature.com. January 7, 2011, accessed May 21, 2015 .
- ↑ E. Pays, B. Vanhollebeke, L. Vanhamme, F. Paturiaux-Hanocq, DP Nolan, D. Pérez-Morga: The trypanolytic factor of human serum. In: Nat Rev Microbiol . 4 (6), Jun 2006, pp. 477-486. PMID 16710327
- ↑ G. Genovese et al: Association of trypanolytic ApoL1 variants with kidney disease in African Americans . In: Science . tape 329 , no. 5993 , August 13, 2010, p. 841-845 , doi : 10.1126 / science.1193032 , PMID 20647424 .
- ↑ S. Mihok, O. Maramba, E. Munyoki, J. Kagoiya: Mechanical transmission of Trypanosoma spp. by African Stomoxyinae (Diptera: Muscidae). In: Trop Med Parasitol. 46 (2), Jun 1995, pp. 103-105. PMID 8525279
- ↑ K. Fenn, KR Matthews: The cell biology of Trypanosoma brucei differentiation. In: Curr Opin Microbiol. 10 (6), Dec 2007, pp. 539-546. PMID 17997129
- ↑ M. Berriman, E. Ghedin, C. Hertz-Fowler et al.: The genome of the African trypanosome Trypanosoma brucei. In: Science. 309 (5733), Jul 15, 2005, pp. 416-422. PMID 16020726
- ^ S. Haile, B. Papadopoulou: Developmental regulation of gene expression in trypanosomatid parasitic protozoa. In: Curr Opin Microbiol. 10 (6), Dec 2007, pp. 569-577. PMID 18177626
- ^ JE Taylor, G. Rudenko: Switching trypanosome coats: what's in the wardrobe? In: Trends Genet. 22 (11), Nov 2006, pp. 614-620. PMID 16908087
- ↑ M. Engstler, T. Pfohl, S. Herminghaus, M. Boshart, G. Wiegertjes, N. Heddergott, P. Overath: Hydrodynamic flow-mediated protein sorting on the cell surface of trypanosomes. In: Cell . 131 (3), Nov 2, 2007, pp. 505-515. PMID 17981118
- ^ Marianne Abele-Horn: Antimicrobial Therapy. Decision support for the treatment and prophylaxis of infectious diseases. With the collaboration of Werner Heinz, Hartwig Klinker, Johann Schurz and August Stich, 2nd, revised and expanded edition. Peter Wiehl, Marburg 2009, ISBN 978-3-927219-14-4 , p. 295.
- ^ Marianne Abele-Horn: Antimicrobial Therapy. Decision support for the treatment and prophylaxis of infectious diseases. With the collaboration of Werner Heinz, Hartwig Klinker, Johann Schurz and August Stich, 2nd, revised and expanded edition. Peter Wiehl, Marburg 2009, ISBN 978-3-927219-14-4 , p. 295.
- ↑ Marianne Abele-Horn (2009), p. 295.
- ↑ a b A. Lüscher, HP de Koning, P. Mäser: Chemotherapeutic strategies against Trypanosoma brucei: drug targets vs. drug targeting. In: Curr Pharm Des . 13 (6), 2007, pp. 555-567. PMID 17346174
literature
- Ian Maudlin, PH Holmes, Michael A. Miles (Eds.): The Trypanosomiases . CABI Publishing, Wallingford 2004 ISBN 0-85199-475-X