Flux balance analysis: Difference between revisions
m Link repair: Metabolic Network Modelling -> Metabolic network modelling - You can help! |
|||
| Line 32: | Line 32: | ||
=== Objective Function=== |
=== Objective Function=== |
||
In FBA there are a large number of mathematically acceptable solutions to the steady-state problem ( |
In FBA there are a large number of mathematically acceptable solutions to the steady-state problem <math>(S\vec v=0)</math> but the ones that are biologically interesting are those that produce the desired metabolites in the correct proportion. The set of metabolites, in the correct proportions, that an FBA model tries to create is called the objective function. When modelling an organism the objective function is generally the biomass of the organism and simulates growth and reproduction. If the biomass function is defined sensibly, or exactly measured experimentally, it can play an important role in making the results of FBA biologically applicable: by ensuring that the correct proportion of metabolites are produced by metabolism and by predicting exact rates of Biomass production for example. |
||
When modelling smaller networks the objective function can be changed accordingly. An example of this would be in the study of the [[Glycolysis |carbohydrate metabolism]] pathways where the objective function would probably be defined as a certain proportion of [[Adenosine triphosphate |ATP]] and [[NADH]] and thus simulate the production of high energy metabolites by this pathway. |
When modelling smaller networks the objective function can be changed accordingly. An example of this would be in the study of the [[Glycolysis |carbohydrate metabolism]] pathways where the objective function would probably be defined as a certain proportion of [[Adenosine triphosphate |ATP]] and [[NADH]] and thus simulate the production of high energy metabolites by this pathway. |
||
Revision as of 14:18, 9 July 2010
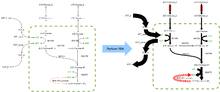
Flux Balance Analysis (FBA) is a mathematical method for analysing metabolism. It does not require knowledge of metabolite concentration or details of the enzyme kinetics of the system. The assumption is made that the system being studied is homeostatic and the technique then aims to answer the question: given some known available nutrients, which set of metabolic fluxes maximises the growth rate of an organism whilst preserving the internal concentration of metabolites?
A notable example of the success of FBA is the ability to accurately predict the growth rate of E. coli when cultured in different conditions[1]. More generally, suitable organisms can be cultivated in media with defined concentrations of nutrients, and their growth rates measured, so that the predictions of FBA can be compared with experiments and the underlying metabolic model corrected accordingly.
A good description of the basic concepts of FBA can be found in the freely available supplementary material to Edwards et al. 2001[2] which can be found at the Nature website[3]. Further sources include the book "Systems Biology" by B. Palsson dedicated to the subject[4] and a useful tutorial and paper by J. Orth[5]. Many other sources of information on the technique exist in published scientific literature including Lee et al. 2006[6] and Feist et al. 2008[7].
Comparison with other techniques
FBA provides a less simplistic analysis than Choke Point Analysis while requiring far less information on reaction rates and a much less complete network reconstruction than a full dynamic simulation would require. In filling this niche, FBA has been shown to be a very useful technique for analysis of the metabolic capabilities of cellular systems.
Choke Point Analysis
Unlike Choke Point Analysis, FBA is a true form of metabolic network modelling because it considers the metabolic network as a single entity (the stoichiometric matrix) at all stages of analysis. This means that network effects, such as chemical reactions in distant pathways affecting each other, can be reproduced in the model. The upside to the inability of choke point analysis to simulate network effects is that it considers each reaction within a network in isolation and thus can suggest important reactions in a network even if a network is highly fragmented and contains many gaps.
Dynamic Metabolic Simulation
Unlike Dynamic Metabolic Simulation, FBA assumes that the internal concentration of metabolites within a system stays constant over time and thus is unable to provide anything other than steady-state solutions. It is unlikely that FBA could, for example, simulate the functioning of a nerve cell. Since the internal concentration of metabolites is not considered within a model, it is possible that an FBA solution could contain metabolites at a concentration too high to be biologically acceptable: a problem that dynamic metabolic simulations would probably avoid. One advantage of the simplicity of FBA over dynamic simulations is that they are far less computationally expensive, allowing the simulation of large numbers of perturbations to the network. A second advantage is that the reconstructed model can be substantially simpler by avoiding the need to consider enzyme rates and the effect of complex interactions on enzyme kinetics.
Model Preparation
A comprehensive guide to creating, preparing and analysing a metabolic model using FBA, in addition to other techniques, was published by Thiele and Palsson in 2010[8]. The key parts of model preparation are: creating a metabolic network without holes, adding constraints to the model and finally adding an objective function (often called the Biomass function), usually to simulate the growth of the organism being modelled.
The Network

Metabolic networks can vary in scope from those describing the metabolism in a single pathway, up to the cell, tissue or organism. The only requirement of a metabolic network that forms the basis of an FBA-ready network is that it contains no gaps. This typically means that extensive manual curation is required, making the preparation of a metabolic network for flux-balance analysis a process that can take months or years. Software packages such as Simpheny[9][10], CellDesigner[11] and MetNetMaker[12], exist to speed up the creation of new FBA-ready metabolic networks.
Generally models are created in BioPAX or SBML format so that further analysis or visualisation can take place in other software although this not a requirement.
Objective Function
In FBA there are a large number of mathematically acceptable solutions to the steady-state problem but the ones that are biologically interesting are those that produce the desired metabolites in the correct proportion. The set of metabolites, in the correct proportions, that an FBA model tries to create is called the objective function. When modelling an organism the objective function is generally the biomass of the organism and simulates growth and reproduction. If the biomass function is defined sensibly, or exactly measured experimentally, it can play an important role in making the results of FBA biologically applicable: by ensuring that the correct proportion of metabolites are produced by metabolism and by predicting exact rates of Biomass production for example.
When modelling smaller networks the objective function can be changed accordingly. An example of this would be in the study of the carbohydrate metabolism pathways where the objective function would probably be defined as a certain proportion of ATP and NADH and thus simulate the production of high energy metabolites by this pathway.
Constraints
A key part of FBA is the ability to add constraints to the flux rates of reactions within networks, forcing them to stay within a range of selected values. This lets the model more accurately simulate real metabolism and can be thought of biologically in two subsets; constraints that limit nutrient uptake and excretion and those that limit the flux through reactions within the organism. FBA-ready metabolic models that have had constraints added can be analysed using software such as the COBRA toolbox[13].
Growth media
Organisms, and all other metabolic systems, require some input of nutrients. Typically the rate of uptake of nutrients is dictated by their availability (a nutrient that isn’t present cannot be absorbed), their concentration and diffusion constants (higher concentrations of quickly-diffusing metabolites are absorbed more quickly) and the method of absorption (such as active transport or facilitated diffusion versus simple diffusion).
If the rate of absorption (and/or excretion) of certain nutrients can be experimentally measured then this information can be added as a constraint on the flux rate at the edges of a metabolic model. This ensures that nutrients that are not present or not absorbed by the organism do not enter its metabolism (the flux rate is constrained to zero) and also means that known nutrient uptake rates are adhered to by the simulation. This provides a secondary method of making sure that the simulated metabolism has experimentally verified properties rather than just mathematically acceptable ones. In mathematical terms, the application of constraints can be considered to reduce the solution space of the FBA model.
Internal constraints
In addition to constraints applied at the edges of a metabolic network, constraints can be applied to reactions deep within the network. These constraints are normally usually simple; they may constrain the direction of a reaction due to energy considerations or constrain the maximum speed of a reaction due to the finite speed of all reaction in nature.
Mathematical Description
A chemical system can be described by a set of differential equations. For instance, suppose a chemical system has two reactions: A + B → C at rate V1 and C → A at rate V2. This system can be described by the differential equations: d(A)/dt = V2 - V1, d(B)/dt = -V1, and d(C)/dt = V1 - V2. A system is at steady state when all three derivatives (rates of change) are 0. FBA involves carrying out a steady state analysis, using the stoichiometric matrix for the system in question.
The system is assumed to be optimized with respect to functions such as maximization of biomass production or minimization of nutrient utilization, following which it is solved to obtain a steady state flux distribution. This flux distribution is then used to interpret the metabolic capabilities of the system. The dynamic mass balance of the metabolic system is described using the stoichiometric matrix, relating the flux rates of enzymatic reactions, to time derivatives of metabolite concentrations, as
where signifies the internal fluxes, represents the exchange fluxes in the system and is the number of external metabolites in the system. At steady state,
Therefore, the required flux distribution belongs to the null space of . Since , the system is under-determined and may be solved for fixing an optimisation criterion, following which, the system translates into a linear programming problem:
where represents the objective function composition, in terms of the fluxes. Further, we can constrain:
which necessitates all internal irreversible reactions to have a flux in the positive direction and allows exchange fluxes to be in either direction. Practically, a finite upper bound can be imposed, so that the problem does not become unbounded. This upper bound may also be decided based on the knowledge of cellular physiology.
Simulating Perturbations


FBA is not computationally intensive, taking on the order of seconds to calculate optimal fluxes for biomass production for a simple organism (around 1000 reactions). This means that the effect of deleting reactions from the network and/or changing flux constraints can be sensibly modelled on a single computer.
Single Reaction Deletion
A frequently used technique to search a metabolic network for reactions that are particularly critical to the production of biomass. By removing each reaction in a network in turn and measuring the predicted flux through the biomass function, each reaction can be classified as either essential (if the flux through the biomass function is substantially reduced) or non-essential (if the flux through the biomass function is unchanged or only slightly reduced).
Reaction Inhibition
The effect of inhibiting a reaction, rather than removing it entirely, can be simulated in FBA by restricting the allowed flux through it. The effect of an inhibition can be classified as lethal or non-lethal by applying the same criteria as in the case of a deletion where a suitable threshold is used to distinguish “substantially reduced” from “slightly reduced”. Generally the choice of threshold is arbitrary but a reasonable estimate can be obtained from growth experiments where the simulated inhibitions/deletions are actually performed and growth rate is measured.
Interpreting Results
The utility of reaction inhibition and deletion analyses is most clear if a gene-protein-reaction matrix has been assembled for the network being studied with FBA. If this has been done then information on which reactions are essential can be converted into information on which genes are essential (and thus what gene defects may cause a certain disease) or which proteins/enzymes are essential (and thus what enzymes are the most promising drug targets in pathogens).
Reaction Deletion in Pairs
An extension of single reaction deletions are double reaction deletions where all possible pairs of reactions are deleted. This can be useful when looking for drug targets as it allows the simulation of multi-target treatments, either by a single drug with multiple targets or by drug combinations.
Growth Media Modification
FBA has also been used to simulate the effect on growth rate of changes in the growth media of the metabolic system being studied. In E. coli the predicted growth rates of bacteria in varying media have been shown to correlate well with experimental results[14] as well as to define precise minimal media for the culture of Salmonella typhimurium[15].
References
- ^ Edwards, J., Ibarra, R. & Palsson, B. In silico predictions of Escherichia coli metabolic capabilities are consistent with experimental data. Nature Biotechnology 19, 125–130(2001).
- ^ Edwards, J., Ibarra, R. & Palsson, B. In silico predictions of Escherichia coli metabolic capabilities are consistent with experimental data. Nature Biotechnology 19, 125–130(2001).
- ^ (http://www.nature.com/nbt/web_extras/supp_info/nbt0201_125/info_frame.html)
- ^ Palsson, B.O. Systems Biology: Properties of Reconstructed Networks. 334(Cambridge University Press: 2006).
- ^ Orth, J.D., Thiele, I. & Palsson, B.Ø. What is flux balance analysis? Nature Biotechnology 28, 245-248(2010).
- ^ Lee, J.M., Gianchandani, E.P. & Papin, J.A. Flux balance analysis in the era of metabolomics. Briefings in bioinformatics 7, 140-50(2006).
- ^ Feist, A.M. & Palsson, B.Ø. The growing scope of applications of genome-scale metabolic reconstructions using Escherichia coli. Nature biotechnology 26, 659-67(2008).
- ^ Thiele, I. & Palsson, B.Ø. A protocol for generating a high-quality genome-scale metabolic reconstruction. Nature protocols 5, 93-121(2010).
- ^ Schilling, C.H. et al. SimPheny™: A Computational Infrastructure for Systems Biology. (2008).
- ^ http://www.genomatica.com/technology/technologySuite.html
- ^ www.celldesigner.org
- ^ http://www.bioinformatics.leeds.ac.uk/~pytf/metnetmaker
- ^ Becker, S.A. et al. Quantitative prediction of cellular metabolism with constraint-based models: the COBRA Toolbox. Nature protocols 2, 727-38(2007).
- ^ Edwards, J., Ibarra, R. & Palsson, B. In silico predictions of Escherichia coli metabolic capabilities are consistent with experimental data. Nature Biotechnology 19, 125–130(2001).
- ^ Raghunathan, A. et al. Constraint-based analysis of metabolic capacity of Salmonella typhimurium during host-pathogen interaction. BMC systems biology 3, 38(2009).





![{\displaystyle \mathbf {v} =[v_{1}\ v_{2}\ ...\ v_{n}\ b_{1}\ b_{2}\ ...\ b_{n_{ext}}]^{T}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/8b99dd56abfa7959c1e852f52f6d2daff4ae9bf2)










